Methodological Assessment of ExoGAG for Isolation of Cerebrospinal Fluid Extracellular Vesicles as a Source of Biomarkers
- PMID: 39769471
- PMCID: PMC11679985
- DOI: 10.3390/ijms252413705
Methodological Assessment of ExoGAG for Isolation of Cerebrospinal Fluid Extracellular Vesicles as a Source of Biomarkers
Abstract
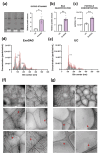
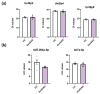
Extracellular vesicles (EVs) in cerebrospinal fluid (CSF) represent a valuable source of biomarkers for central nervous system (CNS) diseases, offering new pathways for diagnosis and monitoring. However, existing methods for isolating EVs from CSF often prove to be labor-intensive and reliant on specialized equipment, hindering their clinical application. In this study, we present a novel, clinically compatible method for isolating EVs from CSF. We optimized the use of ExoGAG, a commercially available reagent that has been tested in plasma, urine and semen, and compared it directly with differential ultracentrifugation using Western blotting, protein quantification, nanoparticle tracking analysis, and cryogenic electron microscopy. Additionally, we analyzed the presence of specific microRNAs (miRNAs) known to be present in CSF-derived EVs. Our data demonstrate that ExoGAG is an effective method for isolating EVs from CSF, yielding a higher amount of EVs compared to traditional ultracentrifugation methods, and with comparable levels of specific miRNAs. In conclusion, the use of ExoGAG in a clinical setting may facilitate the testing of biomarkers essential for tracking brain pathology in CNS diseases.
Keywords: ExoGAG; biomarkers; cerebrospinal fluid; extracellular vesicles; isolation method; miRNAs; ultracentrifugation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hook V., Podvin S., Mosier C., Boyarko B., Seyffert L., Stringer H., Rissman R.A. Emerging evidence for dysregulated proteome cargoes of tau-propagating extracellular vesicles driven by familial mutations of tau and presenilin. Extracell. Vesicles Circ. Nucleic Acids. 2023;4:588–598. doi: 10.20517/evcna.2023.44. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

