Targeted Therapy for Severe Sjogren's Syndrome: A Focus on Mesenchymal Stem Cells
- PMID: 39769474
- PMCID: PMC11677171
- DOI: 10.3390/ijms252413712
Targeted Therapy for Severe Sjogren's Syndrome: A Focus on Mesenchymal Stem Cells
Abstract
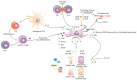
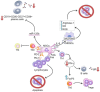
Primary Sjögren's syndrome (pSS) is an autoimmune disease characterized by the infiltration of lymphocytes on salivary and lacrimal glands, resulting in their dysfunction. Patients suffering from severe pSS have an increased risk of developing multi-organ dysfunction syndrome due to the development of systemic inflammatory response, which results in immune cell-driven injury of the lungs, kidneys, liver, and brain. Therapeutic agents that are used for the treatment of severe pSS encounter various limitations and challenges that can impact their effectiveness. Accordingly, there is a need for targeted, personalized therapy that could address the underlying detrimental immune response while minimizing side effects. Results obtained in a large number of recently published studies have demonstrated the therapeutic efficacy of mesenchymal stem cells (MSCs) in the treatment of severe pSS. MSCs, in a juxtacrine and paracrine manner, suppressed the generation of inflammatory Th1 and Th17 lymphocytes, induced the expansion of immunosuppressive cells, impaired the cross-talk between auto-reactive T and B cells, and prevented the synthesis and secretion of auto-antibodies. Additionally, MSC-derived growth and trophic factors promoted survival and prevented apoptosis of injured cells in inflamed lacrimal and salivary glands, thereby enhancing their repair and regeneration. In this review article, we summarized current knowledge about the molecular mechanisms that are responsible for the beneficial effects of MSCs in the suppression of immune cell-driven injury of exocrine glands and vital organs, paving the way for a better understanding of their therapeutic potential in the targeted therapy of severe pSS.
Keywords: autoimmune response; immunosuppression; mesenchymal stem cells; primary Sjögren’s syndrome; targeted therapy.
Conflict of interest statement
Author Carl Randall Harrell was employed by the company Regenerative Processing Plant, LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

