Jeju Citrus (Citrus unshiu) Leaf Extract and Hesperidin Inhibit Small Intestinal α-Glucosidase Activities In Vitro and Postprandial Hyperglycemia in Animal Model
- PMID: 39769483
- PMCID: PMC11679778
- DOI: 10.3390/ijms252413721
Jeju Citrus (Citrus unshiu) Leaf Extract and Hesperidin Inhibit Small Intestinal α-Glucosidase Activities In Vitro and Postprandial Hyperglycemia in Animal Model
Abstract
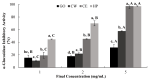
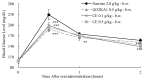
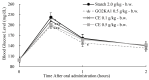
Citrus fruits are widely distributed in East Asia, and tea made from citrus peels has demonstrated health benefits, such as a reduction in fever, inflammation, and high blood pressure. However, citrus leaves have not been evaluated extensively for their possible health benefits. In this study, the α-glucosidase-inhibitory activity of Jeju citrus hot-water (CW) and ethyl alcohol (CE) extracts, along with hesperidin (HP) (a bioactive compound in citrus leaf extracts), was investigated, and furthermore, their effect on postprandial blood glucose reduction in an animal model was determined. The hesperidin contents of CW and CE were 15.80 ± 0.18 and 39.17 ± 0.07 mg/g-extract, respectively. Hesperidin inhibited α-glucosidase (IC50, 4.39), sucrase (0.50), and CE (2.62) and demonstrated higher α-glucosidase inhibitory activity when compared to CW (4.99 mg/mL). When using an SD rat model, during sucrose and starch loading tests with CE (p < 0.01) and HP (p < 0.01), a significant postprandial blood glucose reduction effect was observed when compared to the control. The maximum blood glucose levels (Cmax) of the CE administration group decreased by about 15% (from 229.3 ± 14.5 to 194.0 ± 7.4, p < 0.01) and 11% (from 225.1 ± 13.8 to 201.1 ± 7.2 hr·mg/dL, p < 0.05) in the sucrose and starch loading tests, respectively. Our findings suggest that citrus leaf extracts standardized to hesperidin may reduce postprandial blood glucose levels through the observed inhibitory effect against sucrase, which results in delayed carbohydrate absorption. Our findings provide a biochemical rationale for further evaluating the benefits of citrus leaves.
Keywords: Citrus unshiu leaf; diabetes; inhibition; postprandial hyperglycemia; α-glucosidases.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



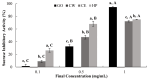
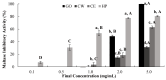
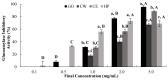


Similar articles
-
The Postprandial Anti-Hyperglycemic Effect of Pyridoxine and Its Derivatives Using In Vitro and In Vivo Animal Models.Nutrients. 2018 Feb 28;10(3):285. doi: 10.3390/nu10030285. Nutrients. 2018. PMID: 29495635 Free PMC article.
-
Selected coffee (Coffea arabica L.) extracts inhibit intestinal α-glucosidases activities in-vitro and postprandial hyperglycemia in SD Rats.BMC Complement Med Ther. 2022 Sep 23;22(1):249. doi: 10.1186/s12906-022-03726-7. BMC Complement Med Ther. 2022. PMID: 36151573 Free PMC article.
-
Selected tea and tea pomace extracts inhibit intestinal α-glucosidase activity in vitro and postprandial hyperglycemia in vivo.Int J Mol Sci. 2015 Apr 21;16(4):8811-25. doi: 10.3390/ijms16048811. Int J Mol Sci. 2015. PMID: 25906471 Free PMC article.
-
A systematic review of the inhibitory effect of extracts from edible parts of nuts on α-glucosidase activity.Food Funct. 2023 Jul 3;14(13):5962-5976. doi: 10.1039/d3fo00328k. Food Funct. 2023. PMID: 37306209
-
An overview on the role of bioactive α-glucosidase inhibitors in ameliorating diabetic complications.Food Chem Toxicol. 2020 Nov;145:111738. doi: 10.1016/j.fct.2020.111738. Epub 2020 Sep 9. Food Chem Toxicol. 2020. PMID: 32916220 Free PMC article. Review.
Cited by
-
Immunogenic cell death biomarkers for sepsis diagnosis and mechanism via integrated bioinformatics.Sci Rep. 2025 May 27;15(1):18575. doi: 10.1038/s41598-025-03282-3. Sci Rep. 2025. PMID: 40425742 Free PMC article.
-
Hypoglycemic Effects of Silphium perfoliatum L. In Vitro and In Vivo and Its Active Composition Identification by UPLC-Triple-TOF-MS/MS.Pharmaceuticals (Basel). 2025 Jul 23;18(8):1087. doi: 10.3390/ph18081087. Pharmaceuticals (Basel). 2025. PMID: 40872480 Free PMC article.
-
α-Glucosidase Inhibition Mechanism and Anti-Hyperglycemic Effects of Flavonoids from Astragali Radix and Their Mixture Effects.Pharmaceuticals (Basel). 2025 May 18;18(5):744. doi: 10.3390/ph18050744. Pharmaceuticals (Basel). 2025. PMID: 40430562 Free PMC article.
-
Harnessing Molecular Phylogeny and Chemometrics for Taxonomic Validation of Korean Aromatic Plants: Integrating Genomics with Practical Applications.Plants (Basel). 2025 Aug 1;14(15):2364. doi: 10.3390/plants14152364. Plants (Basel). 2025. PMID: 40805713 Free PMC article. Review.
References
-
- International Diabetes Federation . IDF Diabetes Atlas 2021. 10th ed. International Diabetes Federation; Brussels, Belgium: 2021. - PubMed
-
- Jo S.H., Cho C.Y., Lee J.Y., Ha K.S., Kwon Y.I., Apostolidis E. In vitro and in vivo reduction of postprandial blood glucose levels by ethyl alcohol and water Zingiber mioga extracts through the inhibition of carbohydrate hydrolyzing enzymes. BMC Complement. Altern. Med. 2016;16:111. doi: 10.1186/s12906-016-1090-4. - DOI - PMC - PubMed
-
- Costabile A., Sarnsamak K., Hauge-Evans A.C. Coffee, type 2 diabetes and pancreatic islet function—A mini-review. J. Funct. Foods. 2018;45:409–416. doi: 10.1016/j.jff.2018.04.011. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

