The Impact of Calcium Overload on Cellular Processes: Exploring Calcicoptosis and Its Therapeutic Potential in Cancer
- PMID: 39769488
- PMCID: PMC11679949
- DOI: 10.3390/ijms252413727
The Impact of Calcium Overload on Cellular Processes: Exploring Calcicoptosis and Its Therapeutic Potential in Cancer
Abstract
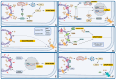
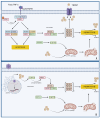
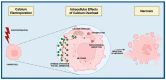
The key role of calcium in various physiological and pathological processes includes its involvement in various forms of regulated cell death (RCD). The concept of 'calcicoptosis' has been introduced as a calcium-induced phenomenon associated with oxidative stress and cellular damage. However, its definition remains controversial within the research community, with some considering it a general form of calcium overload stress, while others view it as a tumor-specific calcium-induced cell death. This review examines 'calcicoptosis' in the context of established RCD mechanisms such as apoptosis, necroptosis, ferroptosis, and others. It further analyzes the intricate relationship between calcium dysregulation and oxidative stress, emphasizing that while calcium overload often triggers cell death, it may not represent an entirely new type of RCD but rather an extension of known pathways. The purpose of this paper is to discuss the implications of this perspective for cancer therapy focusing on calcium-based nanoparticles. By investigating the connections between calcium dynamics and cell death pathways, this review contributes to the advancement of our understanding of calcicoptosis and its possible therapeutic uses.
Keywords: apoptosis; calcicoptosis; calcium; cancer; necroptosis; necrosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Li L., Xin J., Wang H., Wang Y., Peng W., Sun N., Huang H., Zhou Y., Liu X., Lin Y., et al. Fluoride Disrupts Intestinal Epithelial Tight Junction Integrity through Intracellular Calcium-Mediated RhoA/ROCK Signaling and Myosin Light Chain Kinase. Ecotoxicol. Environ. Saf. 2023;257:114940. doi: 10.1016/j.ecoenv.2023.114940. - DOI - PubMed
-
- Jordan M.R., Lopez R.A., Morrisonponce D. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2024. Asystole. - PubMed
-
- Shlisky J., Mandlik R., Askari S., Abrams S., Belizan J.M., Bourassa M.W., Cormick G., Driller-Colangelo A., Gomes F., Khadilkar A., et al. Calcium Deficiency Worldwide: Prevalence of Inadequate Intakes and Associated Health Outcomes. Ann. N. Y. Acad. Sci. 2022;1512:10–28. doi: 10.1111/nyas.14758. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

