Metabolic Biomarkers of Liver Failure in Cell Models and Patient Sera: Toward Liver Damage Evaluation In Vitro
- PMID: 39769500
- PMCID: PMC11677895
- DOI: 10.3390/ijms252413739
Metabolic Biomarkers of Liver Failure in Cell Models and Patient Sera: Toward Liver Damage Evaluation In Vitro
Abstract
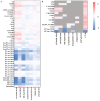
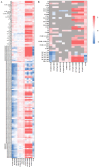
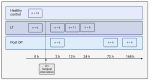
Recent research has concentrated on the development of suitable in vitro cell models for the early identification of hepatotoxicity during drug development in order to reduce the number of animal models and to obtain a better predictability for hepatotoxic reactions in humans. The aim of the presented study was to identify translational biomarkers for acute liver injury in human patients that can serve as biomarkers for hepatocellular injury in vivo and in vitro in simple cell models. Therefore, 188 different metabolites from patients with acute-on-chronic liver failure before and after liver transplantation were analyzed with mass spectrometry. The identified potential metabolic biomarker set, including acylcarnitines, phosphatidylcholines and sphingomyelins, was used to screen primary and permanent hepatocyte culture models for their ability to model hepatotoxic responses caused by different drugs with known and unknown hepatotoxic potential. The results obtained suggest that simple in vitro cell models have the capability to display metabolic responses in biomarkers for liver cell damage in course of the treatment with different drugs and therefore can serve as a basis for in vitro models for metabolic analysis in drug toxicity testing. The identified metabolites should further be evaluated for their potential to serve as a metabolic biomarker set indicating hepatocellular injury in vitro as well as in vivo.
Keywords: drug toxicity testing; hepatocellular injury; in vitro cell models; in vivo–in vitro translation; metabolic biomarker.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Evaluation of multiple mechanism-based toxicity endpoints in primary cultured human hepatocytes for the identification of drugs with clinical hepatotoxicity: Results from 152 marketed drugs with known liver injury profiles.Chem Biol Interact. 2016 Aug 5;255:3-11. doi: 10.1016/j.cbi.2015.11.008. Epub 2015 Nov 12. Chem Biol Interact. 2016. PMID: 26581450
-
In vitro evaluation of hepatotoxic drugs in human hepatocytes from multiple donors: Identification of P450 activity as a potential risk factor for drug-induced liver injuries.Chem Biol Interact. 2016 Aug 5;255:12-22. doi: 10.1016/j.cbi.2015.12.013. Epub 2015 Dec 21. Chem Biol Interact. 2016. PMID: 26718876
-
Toxicant-Induced Metabolic Alterations in Lipid and Amino Acid Pathways Are Predictive of Acute Liver Toxicity in Rats.Int J Mol Sci. 2020 Nov 4;21(21):8250. doi: 10.3390/ijms21218250. Int J Mol Sci. 2020. PMID: 33158035 Free PMC article.
-
Human hepatocytes derived from pluripotent stem cells: a promising cell model for drug hepatotoxicity screening.Arch Toxicol. 2016 Sep;90(9):2049-2061. doi: 10.1007/s00204-016-1756-1. Epub 2016 Jun 20. Arch Toxicol. 2016. PMID: 27325232 Review.
-
Recent advances in three-dimensional cell culturing to assess liver function and dysfunction: from a drug biotransformation and toxicity perspective.Toxicol Mech Methods. 2018 Jun;28(5):369-385. doi: 10.1080/15376516.2017.1422580. Epub 2018 Jan 18. Toxicol Mech Methods. 2018. PMID: 29297242 Review.
References
-
- Sharma A., Nagalli S. Chronic Liver Disease. StatPearls Inc.; Treasure Island, FL, USA: 2022.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

