Positive Effect of Elevated Thawing Rate for Cryopreservation of Human Ovarian Tissue: Transcriptomic Analysis of Fresh and Cryopreserved Cells
- PMID: 39769508
- PMCID: PMC11677892
- DOI: 10.3390/ijms252413747
Positive Effect of Elevated Thawing Rate for Cryopreservation of Human Ovarian Tissue: Transcriptomic Analysis of Fresh and Cryopreserved Cells
Abstract
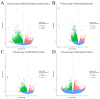
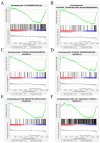
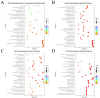
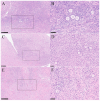
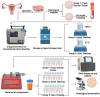
Ovarian tissue cryopreservation has been gradually applied. It is essential to elucidate the differences between cryopreserved and fresh ovarian tissue and to refine cryopreservation protocols for improved outcomes. To explore the transcriptomic differences between fresh ovarian tissue and tissue cryopreserved with an elevated thawing rate. Ovarian tissue samples were collected and cryopreserved (frozen and thawed) following RNA sequencing and histological evaluation. Three groups were formed: fresh tissue (Group 1), frozen tissue after quick thawing at 100 °C (Group 2), and frozen tissue after slow thawing at 37 °C (Group 3). KEGG analysis showed that in comparison with Group 1, DEGs in Group 2 were mainly enriched in the cortisol synthesis and ovarian steroidogenesis pathways, and DEGs in the cells of Group 3 were mainly enriched in the ovarian steroidogenesis pathway. GO analysis showed that compared to cells of Group 2, DEGs in Group 3 were primarily enriched in the SRP-dependent co-translational protein targeting pathway and co-translational protein targeting to the membrane. The results were formulated with a minimal difference in the histological evaluation of cells after quick and slow thawed tissue. Cryopreservation of ovarian tissue by the described method does not decrease follicle production but downregulates the ovarian steroidogenesis pathway, reducing estrogen and progesterone secretion. The quick thawing of ovarian tissue increases the proliferation and apoptosis pathways of cells.
Keywords: Kyoto encyclopedia of genes and genomes (KEGG); RNA sequencing; cryopreservation; differentially expressed genes (DEGs); gene ontology (GO); human ovarian tissue; thawing; transcriptomics.
Conflict of interest statement
Gohar Rahimi was employed by Medizinisches Versorgungszentrum AMEDES für IVF- und Pränatalmedizin in Köln GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Vuković P., Kasum M., Orešković D., Čehić E., Raguž J., Elezaj S., Beketić-Orešković L. Importance of ovarian tissue cryopreservation in fertility preservation and anti-aging treatment. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2019;35:919–923. doi: 10.1080/09513590.2019.1611763. - DOI - PubMed
-
- Fraison E., Huberlant S., Labrune E., Cavalieri M., Montagut M., Brugnon F., Courbiere B. Live birth rate after female fertility preservation for cancer or haematopoietic stem cell transplantation: A systematic review and meta-analysis of the three main techniques; embryo, oocyte and ovarian tissue cryopreservation. Hum. Reprod. 2023;38:489–502. doi: 10.1093/humrep/deac249. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

