Three-Dimensionally Printed Bionic Hydroxyapatite (HAp) Ceramic Scaffolds with Different Structures and Porosities: Strength, Biocompatibility, and Biomedical Application Potential
- PMID: 39769691
- PMCID: PMC11678146
- DOI: 10.3390/ma17246092
Three-Dimensionally Printed Bionic Hydroxyapatite (HAp) Ceramic Scaffolds with Different Structures and Porosities: Strength, Biocompatibility, and Biomedical Application Potential
Abstract
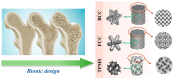
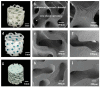
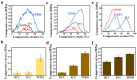
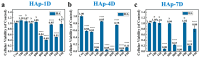
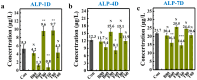
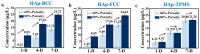
Bionic bioceramic scaffolds are essential for achieving excellent implant properties and biocompatible behavior. In this study, inspired by the microstructure of natural bone, bionic hydroxyapatite (HAp) ceramic scaffolds with different structures (body-centered cubic (BCC), face-centered cubic (FCC), and gyroid Triply Periodic Minimal Surfaces (TPMSs)) and porosities (80 vol.%, 60 vol.%, and 40 vol.%) were designed, 3D-printed, and characterized. The effects of structure and porosity on the morphology, mechanical properties, and in vitro biocompatibility properties of the HAp scaffolds were studied and compared with each other. Interestingly, the HAp scaffold with a porosity of 80 vol.% and a TPMS structure had the best combination of compressive strength and in vitro biocompatibility, and demonstrated a great biomedical application potential for bone repair. We hope this study can provide a reference for the application and development of HAp scaffolds in the field of bone repair engineering.
Keywords: 3D printing; biocompatibility; hydroxyapatite; mechanical properties; scaffold.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Morphologies, mechanical and in vitro behaviors of DLP-based 3D printed HA scaffolds with different structural configurations.RSC Adv. 2023 Jul 11;13(30):20830-20838. doi: 10.1039/d3ra03080f. eCollection 2023 Jul 7. RSC Adv. 2023. PMID: 37441027 Free PMC article.
-
Triply Periodic Minimal Surface-Based Scaffolds for Bone Tissue Engineering: A Mechanical, In Vitro and In Vivo Study.Tissue Eng Part A. 2023 Oct;29(19-20):507-517. doi: 10.1089/ten.TEA.2023.0033. Epub 2023 Jun 19. Tissue Eng Part A. 2023. PMID: 37212290 Free PMC article.
-
3D printed TPMS structural PLA/GO scaffold: Process parameter optimization, porous structure, mechanical and biological properties.J Mech Behav Biomed Mater. 2023 Jun;142:105848. doi: 10.1016/j.jmbbm.2023.105848. Epub 2023 Apr 18. J Mech Behav Biomed Mater. 2023. PMID: 37099921
-
Biomimetic scaffolds using triply periodic minimal surface-based porous structures for biomedical applications.SLAS Technol. 2023 Jun;28(3):165-182. doi: 10.1016/j.slast.2023.04.004. Epub 2023 Apr 29. SLAS Technol. 2023. PMID: 37127136 Review.
-
Applications of Hydroxyapatite-Based Polymeric Scaffolds in Bone Tissue Engineering: An Update.Adv Pharm Bull. 2024 Dec 30;14(4):794-806. doi: 10.34172/apb.43818. Epub 2024 Oct 16. Adv Pharm Bull. 2024. PMID: 40190685 Free PMC article. Review.
References
-
- Li J., Han F., Ma J., Wang H., Pan J., Yang G., Zhao H., Zhao J., Liu J., Liu Z., et al. Targeting endogenous hydrogen peroxide at bone defects promotes bone repair. Adv. Funct. Mater. 2022;32:2111208. doi: 10.1002/adfm.202111208. - DOI
LinkOut - more resources
Full Text Sources

