Nanocomposites Based on Iron Oxide and Carbonaceous Nanoparticles: From Synthesis to Their Biomedical Applications
- PMID: 39769728
- PMCID: PMC11676432
- DOI: 10.3390/ma17246127
Nanocomposites Based on Iron Oxide and Carbonaceous Nanoparticles: From Synthesis to Their Biomedical Applications
Abstract
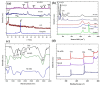
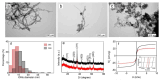
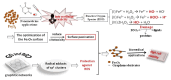
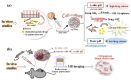
Nanocomposites based on Fe3O4 and carbonaceous nanoparticles (CNPs), including carbon nanotubes (CNTs) and graphene derivatives (graphene oxide (GO) and reduced graphene oxide (RGO)), such as Fe3O4@GO, Fe3O4@RGO, and Fe3O4@CNT, have demonstrated considerable potential in a number of health applications, including tissue regeneration and innovative cancer treatments such as hyperthermia (HT). This is due to their ability to transport drugs and generate localized heat under the influence of an alternating magnetic field on Fe3O4. Despite the promising potential of CNTs and graphene derivatives as drug delivery systems, their use in biological applications is hindered by challenges related to dispersion in physiological media and particle agglomeration. Hence, a solid foundation has been established for the integration of various synthesis techniques for these nanocomposites, with the wet co-precipitation method being the most prevalent. Moreover, the dimensions and morphology of the composite nanoparticles are directly correlated with the value of magnetic saturation, thus influencing the efficiency of the composite in drug delivery and other significant biomedical applications. The current demand for this type of material is related to the loading of a larger quantity of drugs within the hybrid structure of the carrier, with the objective of releasing this amount into the tumor cells. A second demand refers to the biocompatibility of the drug carrier and its capacity to permeate cell membranes, as well as the processes occurring within the drug carriers. The main objective of this paper is to review the synthesis methods used to prepare hybrids based on Fe3O4 and CNPs, such as GO, RGO, and CNTs, and to examinate their role in the formation of hybrid nanoparticles and the correlation between their morphology, the dimensions, and optical/magnetic properties.
Keywords: Fe3O4; anti-cancer therapy; bone tissue engineering; carbon nanotubes; composites; drug delivery; graphene oxide; hyperthermia; nanoparticles; reduced graphene oxide.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Facile synthesis of magnetically separable reduced graphene oxide/magnetite/silver nanocomposites with enhanced catalytic activity.J Colloid Interface Sci. 2015 Dec 1;459:79-85. doi: 10.1016/j.jcis.2015.07.061. Epub 2015 Jul 29. J Colloid Interface Sci. 2015. PMID: 26263498
-
The Influence of Graphene Oxide-Fe3O4 Differently Conjugated with 10-Hydroxycampthotecin and a Rotating Magnetic Field on Adenocarcinoma Cells.Int J Mol Sci. 2024 Jan 11;25(2):930. doi: 10.3390/ijms25020930. Int J Mol Sci. 2024. PMID: 38256006 Free PMC article.
-
Localized cancer treatment by radio-frequency hyperthermia using magnetic nanoparticles immobilized on graphene oxide: from novel synthesis to in vitro studies.J Mater Chem B. 2018 Sep 7;6(33):5385-5399. doi: 10.1039/c8tb01365a. Epub 2018 Aug 13. J Mater Chem B. 2018. PMID: 32254502
-
Photo-fluorescent and magnetic properties of iron oxide nanoparticles for biomedical applications.Nanoscale. 2015 May 14;7(18):8209-32. doi: 10.1039/c5nr01538c. Nanoscale. 2015. PMID: 25899408 Review.
-
Nanoantibiotic effect of carbon-based nanocomposites: epicentric on graphene, carbon nanotubes and fullerene composites: a review.3 Biotech. 2023 May;13(5):147. doi: 10.1007/s13205-023-03552-9. Epub 2023 Apr 27. 3 Biotech. 2023. PMID: 37124988 Free PMC article. Review.
Cited by
-
Influence of Surface Texture in Additively Manufactured Biocompatible Materials and Triboelectric Behavior.Materials (Basel). 2025 Jul 17;18(14):3366. doi: 10.3390/ma18143366. Materials (Basel). 2025. PMID: 40731576 Free PMC article. Review.
-
Improved Biomineralization Using Cellulose Acetate/Magnetic Nanoparticles Composite Membranes.Polymers (Basel). 2025 Jan 15;17(2):209. doi: 10.3390/polym17020209. Polymers (Basel). 2025. PMID: 39861281 Free PMC article.
References
-
- Iijima S. Helical Microtubules of Graphitic Carbon. Nature. 1991;354:56–58. doi: 10.1038/354056a0. - DOI
Publication types
LinkOut - more resources
Full Text Sources

