Heteroleptic Coumarin-Based Silver(I) Complexes: Possible New Antimicrobial Agents
- PMID: 39770006
- PMCID: PMC11678713
- DOI: 10.3390/molecules29245917
Heteroleptic Coumarin-Based Silver(I) Complexes: Possible New Antimicrobial Agents
Abstract
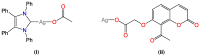
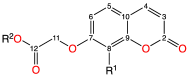
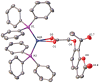
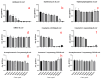
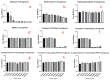
Heteroleptic coumarin-based silver(I) complexes with improved solubility profiles were synthesised using either triphenylphosphine or an N-heterocyclic carbene as adduct ligands, and were fully characterised using IR and NMR spectroscopy, elemental analysis, and, where possible, X-ray crystallography. The triphenylphosphine adducts formed well-resolved structures, where the oxyacetate ligands asymmetrically chelated the silver(I) ion in a bidentate chelating mode, and the silver(I) ion was also bound to two triphenylphosphine ligands. The solubility profile and photostability of the adducts were considerably improved compared to those of previously isolated simple coumarin silver(I) complexes. Analysis of the coumarin N-heterocyclic carbene(NHC) silver(I) adduct indicated that it likely formed as a complex aggregate species with an overall stoichiometry of 1:1:1 coumarin:Ag(I):NHC. The Kirby Bauer assay and broth microdilution assays were used to assess the silver(I) complexes' and adducts' antimicrobial activity against pathogenic strains of Pseudomonas aeruginosa, Escherichia coli, and MRSA. Interestingly, the formation of more soluble complexes did not increase the activity of the silver(I) complexes and, in effect, made them less effective antimicrobial agents, particularly against Escherichia coli and Pseudomonas aeruginosa, although they retained their activity against MRSA.
Keywords: antimicrobial; coumarin; hybrid; photostability; silver(I).
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- UNEP . Bracing for Superbugs: Strengthening Environmental Action in the One Health Response to Antimicrobial Resistance. UNEP; Nairobi, Kenya: 2023.
-
- Mazumder P., Kumar M. Antimicrobial Resistance Ignited by COVID-19 Pandemic: SOS for Antimicrobial Stewardship. In: Kumar M., Kuroda K., Mukherjee S., Ngiehm L.D., Vithanage M., Tyagi V.K., editors. Wastewater Surveillance for COVID-19 Management. Springer International Publishing; Cham, Switzerland: 2024. pp. 323–336.
-
- Sullivan M., Kia A.F.-A., Long M., Walsh M., Kavanagh K., McClean S., Creaven B.S. Isolation and characterisation of silver(I) complexes of substituted coumarin-4-carboxylates which are effective against Pseudomonas aeruginosa biofilms. Polyhedron. 2014;67:549–559. doi: 10.1016/j.poly.2013.09.042. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

