Sphaeropsidin A Loaded in Liposomes to Reduce Its Cytotoxicity and Preserve Antifungal Activity Against Candida auris
- PMID: 39770037
- PMCID: PMC11678014
- DOI: 10.3390/molecules29245949
Sphaeropsidin A Loaded in Liposomes to Reduce Its Cytotoxicity and Preserve Antifungal Activity Against Candida auris
Abstract
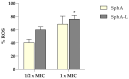
Candida species constitute the most common cause of fungal infections in humans; the emergence of resistance and biofilm formation by Candida species further threaten the limited availability of antifungal agents. Over the past decade, C. auris has caused significant outbreaks worldwide and has emerged as a human pathogenic fungus that causes diseases ranging from superficial to life-threatening disseminated infections. Despite the recent advances in antifungal research, the mechanisms of drug resistance in C. auris remain poorly understood even as its ability to form biofilms poses a significant therapeutic challenge. The purpose of this research was to elucidate the fungal properties of Sphaeropsidin A (SphA), a secondary metabolite derived from Diplodia fungi, with a specific focus on its efficacy against C. auris. This study revealed that SphA and its liposomal encapsulated (SphA-L) form are fungistatic with time-kill kinetics highlighting their efficacy and significantly inhibited the formation of C. auris biofilms. Our investigation into the antifungal mechanism of this drug revealed notable alterations in ROS production and the disruption of the Candida cell cycle. Our findings show that SphA-L impairs key pathogenic traits of C. auris, such as its ability to adhere to human epithelial cell lines, while exhibiting no harmful effects on human cells, highlighting its potential as a future therapeutic agent. In Caenorhabditis elegans infection models, both ShpA and SphA-L displayed effective antifungal activity, significantly reducing the C. auris fungal load and improving nematode survival rates, underscoring their promise as antifungal candidates. Overall, the potent antifungal effects of SphA and SphA-L against C. auris encourage further research.
Keywords: Caenorhabditis elegans; Candida auris; ROS production; Sphaeropsidin A; antifungal activity; biofilm; loaded liposome; nosocomial infection.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

