Bis-Iridoid Glycosides and Triterpenoids from Kolkwitzia amabilis and Their Potential as Inhibitors of ACC1 and ACL
- PMID: 39770069
- PMCID: PMC11678491
- DOI: 10.3390/molecules29245980
Bis-Iridoid Glycosides and Triterpenoids from Kolkwitzia amabilis and Their Potential as Inhibitors of ACC1 and ACL
Abstract
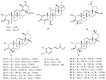
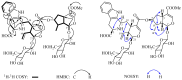
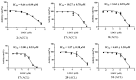
A comprehensive phytochemical investigation of the twigs/leaves and flower buds of Kolkwitzia amabilis, a rare deciduous shrub native to China, led to the isolation of 39 structurally diverse compounds. These compounds include 11 iridoid glycosides (1-4 and 7-13), 20 triterpenoids (5, 6, and 14-31), and 8 phenylpropanoids (32-39). Among these, amabiliosides A (1) and B (2) represent previously undescribed bis-iridoid glycosides, while amabiliosides C (3) and D (4) feature a unique bis-iridoid-monoterpenoid indole alkaloid scaffold with a tetrahydro-β-carboline-5-carboxylic acid moiety. Amabiliacids A (5) and B (6) are 24-nor-ursane-type triterpenoids characterized by an uncommon ∆11,13(18) transannular double bond. Their chemical structures and absolute configurations were elucidated through spectroscopic data and electronic circular dichroism analyses. Compound 2 exhibited a moderate inhibitory effect against acetyl CoA carboxylase 1 (ACC1), with an IC50 value of 9.6 μM. Lonicejaposide C (8), 3β-O-trans-caffeoyl-olean-12-en-28-oic acid (29), and (23E)-coumaroylhederagenin (31) showed notable inhibitory effects on ATP-citrate lyase (ACL), with IC50 values of 3.6, 1.6, and 4.7 μM, respectively. Additionally, 3β-acetyl-ursolic acid (17) demonstrated dual inhibitory activity against both ACC1 and ACL, with IC50 values of 10.3 and 2.0 μM, respectively. The interactions of the active compounds with ACC1 and ACL enzymes were examined through molecular docking studies. From a chemotaxonomic perspective, the isolation of bis-iridoid glycosides in this study may aid in clarifying the taxonomic relationship between the genera Kolkwitzia and Lonicera within the Caprifoliaceae family. These findings highlight the importance of conserving plant species with unique and diverse secondary metabolites, which could serve as potential sources of new therapeutic agents for treating ACC1/ACL-associated diseases.
Keywords: ATP-citrate lyase; Kolkwitzia amabilis; acetyl CoA carboxylase 1; bis-iridoid glycosides; triterpenoids.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Chinese Pharmacopoeia Commission . Pharmacopoeia of the People’s Republic of China Part I. Chinese Medical Science and Technology Press; Beijing, China: 2020.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

