Specific Ion Effects in Hydrogels
- PMID: 39770080
- PMCID: PMC11677444
- DOI: 10.3390/molecules29245990
Specific Ion Effects in Hydrogels
Abstract
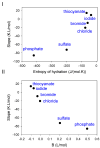
Specific ion effects on the structure and function of many biological macromolecules, their associations, colloidal systems, interfacial phenomena, and even "simple" electrolytes solutions are ubiquitous. The molecular origin of such phenomena is discussed either as a salt-induced change of the water structure (the hydrogen bond network) or some specific (solvent mediated) interactions of one or both of the ions of the electrolyte with the investigated co-solute (macromolecules or colloidal particles). The case of hydrogels is of high interest but is only marginally explored with respect to other physico-chemical systems because they are formed through the interactions of gelling agents in the presence of water and the added electrolyte. In addition, hydrogels in a physiological environment, in which they are used for biomedical applications, may be subjected to fluctuations in their ionic environment. In this review, specific ion effects on the properties of hydrogels (made from macromolecules or small-molecular-weight gelators) are reviewed and discussed. In particular, the importance of specific ion binding to the molecules constituting the gel network versus the effect of the same ions on the structure of water is discussed.
Keywords: chaotropes; hydrogels; kosmotropes; specific binding; specific salt effects.
Conflict of interest statement
The author declares no conflicts of interest.
Figures





References
-
- Hofmeister F. Zur Lehre von der Wirkung der Saltze. Arch. Exp. Pathol. Pharmakol. 1888;24:247–260. doi: 10.1007/BF01918191. - DOI
-
- Marcus Y. Specific ion effects on the surface tension and surface potential of aqueous electrolytes. Curr. Opin. Colloid Interface Sci. 2016;23:94–99. doi: 10.1016/j.cocis.2016.06.016. - DOI
-
- Baglioni P., Giorgi R., Chelazzi D. The degradation of wall paintings and stone: Specific ion effects. Curr. Opin. Colloid Interface Sci. 2016;23:66–71. doi: 10.1016/j.cocis.2016.06.011. - DOI
Publication types
LinkOut - more resources
Full Text Sources

