Nickel-Catalyzed Reductive Cyanation of Aryl Halides and Epoxides with Cyanogen Bromide
- PMID: 39770100
- PMCID: PMC11678332
- DOI: 10.3390/molecules29246016
Nickel-Catalyzed Reductive Cyanation of Aryl Halides and Epoxides with Cyanogen Bromide
Abstract
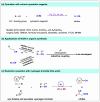
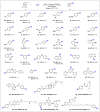
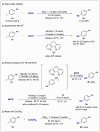
Nitriles are valuable compounds because they have widespread applications in organic chemistry. This report details the nickel-catalyzed reductive cyanation of aryl halides and epoxides with cyanogen bromide for the synthesis of nitriles. This robust protocol underscores the practicality of using a commercially available and cost-effective cyanation reagent. A variety of aryl halides and epoxides featuring diverse functional groups, such as -TMS, -Bpin, -OH, -NH2, -CN, and -CHO, were successfully converted into nitriles in moderate-to-good yields. Moreover, the syntheses at gram-scale and application in late-stage cyanation of natural products and drugs reinforces its potentiality.
Keywords: cyanation; cyanogen bromide; nickel catalysis; nitriles; reductive coupling.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Tathe A.B., Gupta V.D., Sekar N. Synthesis and Combined Experimental and Computational Investigations on Spectroscopic and Photophysical Properties of Red Emitting 3-styryl Coumarins. Dyes Pigment. 2015;119:49–55. doi: 10.1016/j.dyepig.2015.03.023. - DOI
-
- Jones L.H., Summerhill N.W., Swain N.A., Mills J.E. Aromatic Chloride to Nitrile Transformation: Medicinal and Synthetic Chemistry. Med. Chem. Commun. 2010;1:309–318. doi: 10.1039/C0MD00135J. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

