Regioselective Nucleophilic Aromatic Substitution: Theoretical and Experimental Insights into 4-Aminoquinazoline Synthesis as a Privileged Structure in Medicinal Chemistry
- PMID: 39770108
- PMCID: PMC11678402
- DOI: 10.3390/molecules29246021
Regioselective Nucleophilic Aromatic Substitution: Theoretical and Experimental Insights into 4-Aminoquinazoline Synthesis as a Privileged Structure in Medicinal Chemistry
Abstract
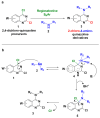
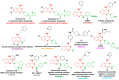
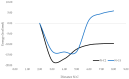
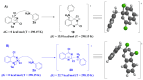
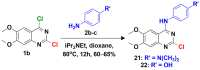
The 4-aminoquinazoline scaffold is a privileged structure in medicinal chemistry. Regioselective nucleophilic aromatic substitution (SNAr) for replacing the chlorine atom at the 4-position of 2,4-dichloroquinazoline precursors is well documented in the scientific literature and has proven useful in synthesizing 2-chloro-4-aminoquinazolines and/or 2,4-diaminoquinazolines for various therapeutic applications. While numerous reports describe reaction conditions involving different nucleophiles, solvents, temperatures, and reaction times, discussions on the regioselectivity of the SNAr step remain scarce. In this study, we combined DFT calculations with 2D-NMR analysis to characterize the structure and understand the electronic factors underlying the regioselective SNAr of 2,4-dichloroquinazolines for the synthesis of bioactive 4-aminoquinazolines. DFT calculations revealed that the carbon atom at the 4-position of 2,4-dichloroquinazoline has a higher LUMO coefficient, making it more susceptible to nucleophilic attack. This observation aligns with the calculated lower activation energy for nucleophilic attack at this position, supporting the regioselectivity of the reaction. To provide guidance for the structural confirmation of 4-amino-substituted product formation when multiple regioisomers are possible, we employed 2D-NMR methods to verify the 4-position substitution pattern in synthesized bioactive 2-chloro-4-aminoquinazolines. These findings are valuable for future research, as many synthetic reports assume regioselective outcomes without sufficient experimental verification.
Keywords: NMR spectroscopy; aminoquinazoline; density functional calculations; medicinal chemistry; nucleophilic substitution; regioselectivity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Sirisoma N., Kasibhatla S., Pervin A., Zhang H., Jiang S., Willardsen J.A., Anderson M.B., Baichwal V., Mather G.G., Jessing K., et al. Discovery of 2-Chloro-N-(4-Methoxyphenyl)-N-Methylquinazolin-4-Amine (EP128265, MPI-0441138) as a Potent Inducer of Apoptosis with High In Vivo Activity. J. Med. Chem. 2008;51:4771–4779. doi: 10.1021/jm8003653. - DOI - PubMed
-
- Wang S.-B., Cui M.-T., Wang X.-F., Ohkoshi E., Goto M., Yang D.-X., Li L., Yuan S., Morris-Natschke S.L., Lee K.-H., et al. Synthesis, Biological Evaluation, and Physicochemical Property Assessment of 4-Substituted 2-Phenylaminoquinazolines as Mer Tyrosine Kinase Inhibitors. Bioorg. Med. Chem. 2016;24:3083–3092. doi: 10.1016/j.bmc.2016.05.025. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 001/Coordenação de Aperfeicoamento de Pessoal de Nível Superior
- CNPq 465.249/2014-0/National Institute of Science and Technology of Drugs and Medicines
- 465.249/2014-0/National Council for Scientific and Technological Development
- E-26/210.718/2024; SEI-260003/006052/2024/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
- E-26/010.000090/2018/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
LinkOut - more resources
Full Text Sources
Research Materials

