Search for New Compounds with Anti-Inflammatory Activity Among 1,2,4-Triazole Derivatives
- PMID: 39770124
- PMCID: PMC11677506
- DOI: 10.3390/molecules29246036
Search for New Compounds with Anti-Inflammatory Activity Among 1,2,4-Triazole Derivatives
Abstract
Compounds containing the 1,2,4-triazole moiety in their structure exhibit broad biological activities. Many of these compounds demonstrate anti-inflammatory activity in vitro through various mechanisms, such as inhibiting COX-1/COX-2 and LOX, modulating pro-inflammatory cytokine levels, or having effects on other specific enzymes. Some also display activities in vivo. In many publications, the activities of new 1,2,4-triazole-based compounds exceed those of the reference drugs, suggesting their promising potential as new therapeutic agents. This review of active 1,2,4-triazole derivatives with anti-inflammatory activity is based on literature published from 2015-2024.
Keywords: 1,2,4-triazole; activity in vitro; activity in vivo; anti-inflammatory activity; cyclooxygenase inhibitors; lipoxygenase inhibitors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

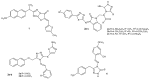
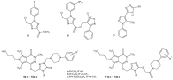
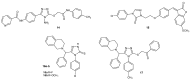


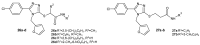
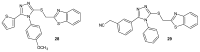

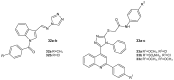
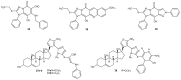


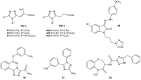
References
-
- Curtis A.D.M., Jennings N. Comprehensive Heterocyclic Chemistry III. Volume 5. Elsevier Ltd.; Amsterdam, The Netherlands: 2008. 1,2,4-Triazoles. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

