Benzylic C-H Oxidation: Recent Advances and Applications in Heterocyclic Synthesis
- PMID: 39770135
- PMCID: PMC11678705
- DOI: 10.3390/molecules29246047
Benzylic C-H Oxidation: Recent Advances and Applications in Heterocyclic Synthesis
Abstract
Benzylic C-H oxidation to form carbonyl compounds, such as ketones, is a fundamental transformation in organic synthesis as it allows for the preparation of versatile intermediates. In this review, we highlight the synthesis of aromatic ketones via catalytic, electrochemical, and photochemical oxidation of alkylarenes using different catalysts and oxidants in the past 5 years. Additionally, we also discuss the synthesis of heterocyclic molecules using benzylic C-H oxidation as a key step. These methods can potentially be used in medicinal, synthetic, and inorganic chemistry.
Keywords: alkylarenes; benzylic C–H oxidation; heterocycles; imidazoles; ketones; oxazoles; quinoxalines; review.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
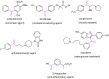


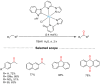
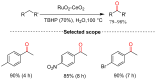
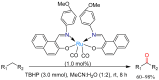












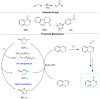
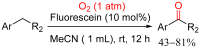





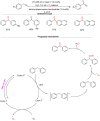




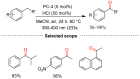
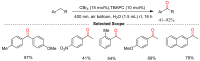




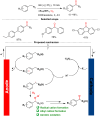
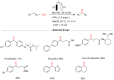
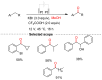
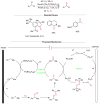







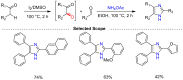


References
-
- Bindu V.H., Parvathaneni S.P., Rao V.J. Iodosobenzoic acid (IBA) catalysed benzylic and aromatic C–H oxidations. Catal. Lett. 2017;147:1434–1440. doi: 10.1007/s10562-017-2050-4. - DOI
-
- Tan J., Zheng T., Yu Y., Xu K. TBHP-promoted direct oxidation reaction of benzylic Csp3–H bonds to ketones. RSC Adv. 2017;7:15176–15180. doi: 10.1039/C7RA00352H. - DOI
-
- Karakaya I., Rizwan K., Munir S. Transition-Metal Catalyzed Coupling Reactions for the Synthesis of (Het) aryl Ketones. An Approach from their Synthesis to Biological Perspectives. ChemistrySelect. 2023;8:e202204005. doi: 10.1002/slct.202204005. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

