Porphyrin-Based Supramolecular Self-Assemblies: Construction, Charge Separation and Transfer, Stability, and Application in Photocatalysis
- PMID: 39770151
- PMCID: PMC11676642
- DOI: 10.3390/molecules29246063
Porphyrin-Based Supramolecular Self-Assemblies: Construction, Charge Separation and Transfer, Stability, and Application in Photocatalysis
Abstract
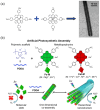
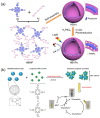
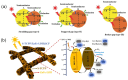
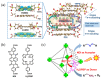
As a key means to solve energy and environmental problems, photocatalytic technology has made remarkable progress in recent years. Organic semiconductor materials offer structural diversity and tunable energy levels and thus attracted great attention. Among them, porphyrin and its derivatives show great potential in photocatalytic reactions and light therapy due to their unique large-π conjugation structure, high apparent quantum efficiency, tailorable functionality, and excellent biocompatibility. Compared to unassembled porphyrin molecules, supramolecular porphyrin assemblies facilitate the solar light absorption and improve the charge transfer and thus exhibit enhanced photocatalytic performance. Herein, the research progress of porphyrin-based supramolecular assemblies, including the construction, the regulation of charge separation and transfer, stability, and application in photocatalysis, was systematically reviewed. The construction strategy of porphyrin supramolecules, the mechanism of charge separation, and the intrinsic relationship of assembling structure-charge transfer-photocatalytic performance received special attention. Surfactants, peptide molecules, polymers, and metal ions were introduced to improve the stability of the porphyrin assemblies. Donor-acceptor structure and co-catalysts were incorporated to inhibit the recombination of the photoinduced charges. These increase the understanding of the porphyrin supramolecules and provide ideas for the design of high-performance porphyrin-based photocatalysts.
Keywords: application; intrinsic relationship between the supramolecular structure and property; mechanism for charge separation; porphyrin; supramolecular photocatalyst.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures















References
-
- Wang Z., Fan J., Cheng B., Yu J., Xu J. Nickel-based cocatalysts for photocatalysis: Hydrogen evolution, overall water splitting and CO2 reduction. Mater. Today Phys. 2020;15:100279. doi: 10.1016/j.mtphys.2020.100279. - DOI
-
- Zhong Q., Zhang Z., Wang H., Zhang X., Wang Y., Wang P., Ma F., Yue Q., Du T., Chen W.-Q., et al. Incorporating scarcity into footprints reveals diverse supply chain hotspots for global fossil fuel management. Appl. Energy. 2023;349:121692. doi: 10.1016/j.apenergy.2023.121692. - DOI
-
- Zhao Y., Jia X., Waterhouse G.I., Wu L.Z., Tung C.H., O’Hare D., Zhang T. Layered double hydroxide nanostructured photocatalysts for renewable energy production. Adv. Energy Mater. 2016;6:1501974. doi: 10.1002/aenm.201501974. - DOI
-
- Yaghoubi S., Mousavi S.M., Babapoor A., Binazadeh M., Lai C.W., Althomali R.H., Rahman M.M., Chiang W.-H. Photocatalysts for solar energy conversion: Recent advances and environmental applications. Renew. Sustain. Energy Rev. 2024;200:114538. doi: 10.1016/j.rser.2024.114538. - DOI
-
- Cao H., Liu F., Tai Y., Wang W., Li X., Li P., Zhao H., Xia Y., Wang S. Promoting photocatalytic performance of TiO2 nanomaterials by structural and electronic modulation. Chem. Eng. J. 2023;466:143219. doi: 10.1016/j.cej.2023.143219. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

