Melittin-Induced Structural Transformations in DMPG and DMPS Lipid Membranes: A Langmuir Monolayer and AFM Study
- PMID: 39770152
- PMCID: PMC11677270
- DOI: 10.3390/molecules29246064
Melittin-Induced Structural Transformations in DMPG and DMPS Lipid Membranes: A Langmuir Monolayer and AFM Study
Abstract
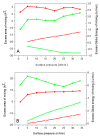
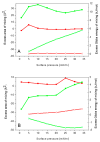
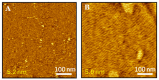
In this study, we explore the interactions between melittin, a cationic antimicrobial peptide, and model lipid membranes composed of the negatively charged phospholipids 1,2-dimyristoyl-sn-glycero-3-phosphoglycerol (DMPG) and 1,2-dimyristoyl-sn-glycero-3-phosphoserine (DMPS). Using the Langmuir monolayer technique and atomic force microscopy (AFM), we reveal novel insights into these interactions. Our key finding is the observation of the ripple phase in the DMPS bilayer on mica, a phenomenon not previously reported for negatively charged single bilayers. This discovery is significant given the critical role of phosphatidylserine (PS) in cancer biology and the potential of melittin as an anticancer agent. We also highlight the importance of subphase composition, as melittin interacts preferentially with lipids in the liquid-condensed phase; thus, selecting the appropriate subphase composition is crucial because it affects lipid behavior and consequently melittin interactions. Our results show that melittin incorporates into lipid monolayers in both liquid-expanded and liquid-condensed phases, enhancing membrane fluidity and disorder, but is expelled from DMPS in the solid phase. AFM imaging further reveals that melittin induces substantial structural changes in the DMPG membrane and forms the ripple phase in the DMPS bilayers. Despite these alterations, melittin does not cause pore formation or membrane rupture, suggesting strong electrostatic adsorption on the membrane surface that prevents penetration. These findings highlight the differential impacts of melittin on lipid monolayers and bilayers and underscore its potential for interacting with membranes without causing disruption.
Keywords: antimicrobial peptides; melittin; model lipid membranes; ripple phase.
Conflict of interest statement
The author declares no conflicts of interest.
Figures



References
-
- Jalalifar S., Razavi S., Mirzaei R., Irajian G., Pooshang Bagheri K. A Hope for Ineffective Antibiotics to Return to Treatment: Investigating the Anti-Biofilm Potential of Melittin Alone and in Combination with Penicillin and Oxacillin against Multidrug Resistant-MRSA and -VRSA. Front. Microbiol. 2024;14:1269392. doi: 10.3389/fmicb.2023.1269392. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

