Intestinal Cells-on-Chip for Permeability Studies
- PMID: 39770217
- PMCID: PMC11679574
- DOI: 10.3390/mi15121464
Intestinal Cells-on-Chip for Permeability Studies
Abstract
Background: To accurately measure permeability of compounds in the intestine, there is a need for preclinical in vitro models that accurately represent the specificity, integrity and complexity of the human small intestinal barrier. Intestine-on-chip systems hold considerable promise as testing platforms, but several characteristics still require optimization and further development.
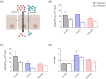
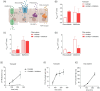
Methods: An established intestine-on-chip model for tissue explants was adopted for intestinal cell monolayer culture. A 3D-printed culture disc was designed to allow cell culture in static conditions and subsequent permeability studies in a dynamic environment. Membrane characteristics and standardized read-outs were investigated and compared to traditional permeability studies under static conditions.
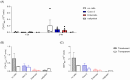
Results: By starting cultures outside the chip in conventional wells plates, the new cell disc design could support accurate cell monolayer formation for both Caco-2 and human enteroids. When transferred to the chip with laminar flow, there was accurate detection of barrier integrity (FD4 and Cascade Blue) and permeability (atenolol/antipyrine). Both flow and membrane characteristics had a significant impact on permeability outcomes.
Conclusions: This novel intestinal cell-on-chip system offers large flexibility for intestinal permeability studies, although it still requires validation with more compounds to reveal its full potential.
Keywords: cell monolayer; in vitro model; intestinal absorption; intestinal barrier; intestine-on-chip; permeability.
Conflict of interest statement
The authors declare no conflicts of interest. Hossein Eslami Amirabadi is employee of AZAR Innovations, and ARTIC Technologies B.V.; Markus Walles and Birk Poller are employees of Novartis Pharma AG. The paper reflects the views of the scientists, and not the company.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

