Differential TLR-ERK1/2 Activity Promotes Viral ssRNA and dsRNA Mimic-Induced Dysregulated Immunity in Macrophages
- PMID: 39770293
- PMCID: PMC11676137
- DOI: 10.3390/pathogens13121033
Differential TLR-ERK1/2 Activity Promotes Viral ssRNA and dsRNA Mimic-Induced Dysregulated Immunity in Macrophages
Abstract
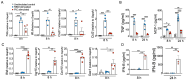
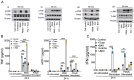
RNA virus-induced excessive inflammation and impaired antiviral interferon (IFN-I) responses are associated with severe disease. This innate immune response, also referred to as "dysregulated immunity" is caused by viral single-stranded RNA (ssRNA)- and double-stranded-RNA (dsRNA)-mediated exuberant inflammation and viral protein-induced IFN antagonism. However, key host factors and the underlying mechanism driving viral RNA-mediated dysregulated immunity are poorly defined. Here, using viral ssRNA and dsRNA mimics, which activate toll-like receptor 7 (TLR7) and TLR3, respectively, we evaluated the role of viral RNAs in causing dysregulated immunity. We observed that murine bone marrow-derived macrophages (BMDMs), when stimulated with TLR3 and TLR7 agonists, induced differential inflammatory and antiviral cytokine response. TLR7 activation triggered a robust inflammatory cytokine/chemokine induction compared to TLR3 activation, whereas TLR3 stimulation induced significantly increased IFN/IFN stimulated gene (ISG) response relative to TLR7 activation. To define the mechanistic basis for dysregulated immunity, we examined cell-surface and endosomal TLR levels and downstream mitogen-activated protein kinase (MAPK) and nuclear factor kappa B (NF-kB) activation. We identified significantly higher cell-surface and endosomal TLR7 levels compared to TLR3, which were associated with early and robust MAPK (p-ERK1/2, p-P38, and p-JNK) and NF-kB activation in TLR7-stimulated macrophages. Furthermore, blocking EKR1/2 and NF-kB activity reduced TLR3/7-induced inflammatory cytokine/chemokine levels, whereas only ERK1/2 inhibition enhanced viral RNA mimic-induced IFN/ISG responses. Collectively, our results illustrate that high cell-surface and endosomal TLR7 expression and robust ERK1/2 activation drive viral ssRNA mimic-induced excessive inflammatory and reduced IFN/ISG response and blocking ERK1/2 activity would likely mitigate viral-RNA/TLR-induced dysregulated immunity.
Keywords: ERK1/2; SARS-CoV-2; TLRs; inflammation; interferon; macrophages.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

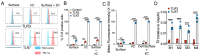


Update of
-
Differential TLR-ERK1/2 activity promotes viral ssRNA and dsRNA mimic-induced dysregulated immunity in macrophages.bioRxiv [Preprint]. 2024 May 25:2024.05.24.595760. doi: 10.1101/2024.05.24.595760. bioRxiv. 2024. Update in: Pathogens. 2024 Nov 23;13(12):1033. doi: 10.3390/pathogens13121033. PMID: 38826464 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

