Use of a Novel Feeding System to Assess the Survival of a Very Stable Mammalian Virus, Porcine Parvovirus, Within Black Soldier Fly (Hermetia illucens) Larvae: A Comparison with Mealworm (Tenebrio molitor) Larvae
- PMID: 39770298
- PMCID: PMC11728632
- DOI: 10.3390/pathogens13121038
Use of a Novel Feeding System to Assess the Survival of a Very Stable Mammalian Virus, Porcine Parvovirus, Within Black Soldier Fly (Hermetia illucens) Larvae: A Comparison with Mealworm (Tenebrio molitor) Larvae
Abstract
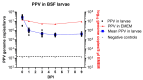
Insect larvae production offers the potential for large-scale synthesis of high-quality protein that can be used as feed or food. However, currently, there are limitations on the source of substrates for the insect larvae to use. One concern is the potential survival of animal pathogens within insect larvae if their feed is contaminated. In this study, the survival of a very stable virus, porcine parvovirus (PPV), within mealworm (Tenebrio molitor) and black soldier fly (BSF) (Hermetia illucens) larvae has been analyzed after oral ingestion of the virus. PPV genomic DNA could be readily detected by PCR in both species of larvae up until 9 days post ingestion (DPI), the end of the study period. Furthermore, infection of susceptible PK15 cells by PPV from homogenized mealworm larvae could be detected until at least 3 DPI, using an immunoperoxidase staining method and, up until 9 DPI, with a more sensitive real time PCR assay. Thus, PPV can remain infectious within mealworm larvae during their main growth phase through to their harvesting. However, it may be considered that PPV is exceptional in this respect since it displays unusual stability, e.g., to heat.
Keywords: insect larvae; virus infectivity assays; virus ingestion; virus survival.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
-
- Van Huis A., Van Itterbeeck J., Klunder H., Mertens E., Halloran A., Muir G., Vantomme P. Edible insects: Future Prospects for Food and Feed Security. Food and Agriculture Organization of the United Nations Forestry Paper; No. 171. Food and Agriculture Organization of the United Nations (FAO); Rome, Italy: 2013. [(accessed on 25 October 2024)]. Available online: https://edepot.wur.nl/258042.
-
- EFSA Scientific Committee Scientific Opinion on a risk profile related to production and consumption of insects as food and feed. EFSA J. 2015;13:4257. doi: 10.2903/j.efsa.2015.4257. - DOI
-
- Lecocq A., Olesen A.S., Lazov C.M., Rajiuddin S.M., Jensen A.B., Lohse L., Rasmussen T.B., Belsham G.J., Bøtner A. Bioexposure assays to assess uptake and survival of viruses in mealworm (Tenebrio molitor) and black soldier fly (Hermetia illucens) larvae. J. Insects Food Feed. 2023;9:1165–1175. doi: 10.3920/JIFF2022.0167. - DOI
-
- Olesen A.S., Lazov C.M., Lecocq A., Accensi F., Jensen A.B., Lohse L., Rasmussen T.B., Belsham G.J., Bøtner A. Uptake and Survival of African Swine Fever Virus in Mealworm (Tenebrio molitor) and Black Soldier Fly (Hermetia illucens) Larvae. Pathogens. 2022;12:47. doi: 10.3390/pathogens12010047. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

