Functional Analysis of Promoters, mRNA Cleavage, and mRNA Secondary Structure on esxB-esxA in Mycolicibacterium smegmatis
- PMID: 39770301
- PMCID: PMC11728522
- DOI: 10.3390/pathogens13121041
Functional Analysis of Promoters, mRNA Cleavage, and mRNA Secondary Structure on esxB-esxA in Mycolicibacterium smegmatis
Abstract
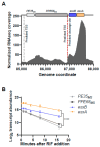
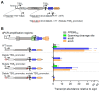
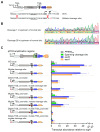
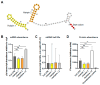
The ESX-1 secretion system is critical for the virulence of Mycobacterium tuberculosis as well as for conjugation in the saprophytic model Mycolicibacterium smegmatis. EsxB (CFP-10) and EsxA (ESAT-6) are secreted effectors required for the function of ESX-1 systems. While some transcription factors regulating the expression of esxB and esxA have been identified, little work has addressed their promoter structures or other determinants of their expression. Here, we defined two promoters, one located two genes upstream of esxB and one located immediately upstream, that contribute substantially to the expression of esxB and esxA. We also defined an mRNA cleavage site within the esxB 5' untranslated region (UTR) and found that a single-nucleotide substitution reprogramed the position of this cleavage event without impacting esxB-esxA transcript abundance. We furthermore investigated the impact of a double stem-loop structure in the esxB 5' UTR and found that it does not confer stability on a reporter gene transcript. Consistent with this, there was no detectable correlation between mRNA half-life and secondary structure near the 5' ends of 5' UTRs on a transcriptome-wide basis. Collectively, these data shed light on the determinants of esxB-esxA expression in M. smegmatis as well as provide broader insight into the determinants of mRNA cleavage in mycobacteria and the relationship between 5' UTR secondary structure and mRNA stability.
Keywords: 5′ UTR; ESX-1; EsxA; EsxB; Mycobacterium smegmatis; mRNA cleavage; mRNA processing; mRNA stability; mycobacteria; promoter; transcription start site; transcriptional regulation; type VII secretion system.
Conflict of interest statement
The authors declare no conflicts of interest. The sponsors had no role in the design, execution, interpretation, or writing of this study.
Figures


References
-
- World Health Organization . Global Tuberculosis Report. WHO; Geneva, Switzerland: 2023.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

