Colistin Resistance Mechanism and Management Strategies of Colistin-Resistant Acinetobacter baumannii Infections
- PMID: 39770308
- PMCID: PMC11728550
- DOI: 10.3390/pathogens13121049
Colistin Resistance Mechanism and Management Strategies of Colistin-Resistant Acinetobacter baumannii Infections
Abstract
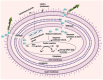
The emergence of antibiotic-resistant Acinetobacter baumannii (A. baumannii) is a pressing threat in clinical settings. Colistin is currently a widely used treatment for multidrug-resistant A. baumannii, serving as the last line of defense. However, reports of colistin-resistant strains of A. baumannii have emerged, underscoring the urgent need to develop alternative medications to combat these serious pathogens. To resist colistin, A. baumannii has developed several mechanisms. These include the loss of outer membrane lipopolysaccharides (LPSs) due to mutation of LPS biosynthetic genes, modification of lipid A (a constituent of LPSs) structure through the addition of phosphoethanolamine (PEtN) moieties to the lipid A component by overexpression of chromosomal pmrCAB operon genes and eptA gene, or acquisition of plasmid-encoded mcr genes through horizontal gene transfer. Other resistance mechanisms involve alterations of outer membrane permeability through porins, the expulsion of colistin by efflux pumps, and heteroresistance. In response to the rising threat of colistin-resistant A. baumannii, researchers have developed various treatment strategies, including antibiotic combination therapy, adjuvants to potentiate antibiotic activity, repurposing existing drugs, antimicrobial peptides, nanotechnology, photodynamic therapy, CRISPR/Cas, and phage therapy. While many of these strategies have shown promise in vitro and in vivo, further clinical trials are necessary to ensure their efficacy and widen their clinical applications. Ongoing research is essential for identifying the most effective therapeutic strategies to manage colistin-resistant A. baumannii. This review explores the genetic mechanisms underlying colistin resistance and assesses potential treatment options for this challenging pathogen.
Keywords: A. baumannii; antibiotic-resistant; colistin; lipid A; mutation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Jiang M., Chen X., Liu S., Zhang Z., Li N., Dong C., Zhang L., Wu H., Zhao S. Epidemiological Analysis of Multidrug-Resistant Acinetobacter baumannii Isolates in a Tertiary Hospital Over a 12-Year Period in China. Front. Public Health. 2021;9:707435. doi: 10.3389/fpubh.2021.707435. - DOI - PMC - PubMed
-
- WHO . WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance. World Health Organization; Geneva, Switzerland: 2024.
-
- CDC . Antibiotic Resistance Threats in the United States. Department of Health and Human Services, CDC; Atlanta, GA, USA: 2019. - DOI
-
- Serapide F., Guastalegname M., Gullì S.P., Lionello R., Bruni A., Garofalo E., Longhini F., Trecarichi E.M., Russo A. Antibiotic Treatment of Carbapenem-Resistant Acinetobacter baumannii Infections in View of the Newly Developed β-Lactams: A Narrative Review of the Existing Evidence. Antibiotics. 2024;13:506. doi: 10.3390/antibiotics13060506. - DOI - PMC - PubMed
-
- Oh M.H., Kim N., Islam M.M., Kim S.Y., Lee D.E., Kim Y.K., Kwon K.T., Lee J.C. Comparative genomic and phenotypic analysis of low- and high-virulent Acinetobacter baumannii strains: Insights into antimicrobial resistance and virulence potential. Microb. Pathog. 2025;198:107118. doi: 10.1016/j.micpath.2024.107118. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

