Vaccination Schedule and Age Influence Impaired Responsiveness to Hepatitis B Vaccination: A Randomized Trial in Central Asia
- PMID: 39770341
- PMCID: PMC11728755
- DOI: 10.3390/pathogens13121082
Vaccination Schedule and Age Influence Impaired Responsiveness to Hepatitis B Vaccination: A Randomized Trial in Central Asia
Abstract
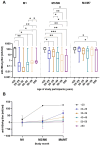
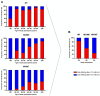
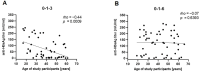
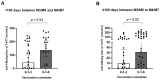
Vaccination against hepatitis B virus (HBV) is the most cost-efficient measure to prevent infection. Still, vaccination coverage among adults in Central Asia, including Kyrgyzstan, remains suboptimal, and data about immune responses to HBV vaccination are lacking. HBV vaccination is given as three injections, whereby the second and third doses are given 1 and 6 months after the first (0-1-6 scheme). However, compliance with the third dose is low in Kyrgyzstan, presumably due to the long time interval between the second and third doses, suggesting that a shortened vaccination schedule could result in better adherence and increased seroconversion. Thus, we conducted a randomized trial of individuals aged 17-66 years comparing the 0-1-6 scheme against a shorter 0-1-3 scheme. Primary outcome measures were post-vaccination titers and the percentage of participants with protective post-vaccination titers (≥10 mIU/mL). Compliance with the completeness of blood draws and administered third vaccine dose was better with the 0-1-3 scheme than with the 0-1-6 scheme. In both study arms combined, younger age (<40 years) was associated with better vaccine protection. The 0-1-6 scheme resulted in higher post-vaccination titers (52 versus 15 mIU/mL, p = 0.002) and a higher seroprotection rate (85% versus 64%, p = 0.01) than the 0-1-3 scheme, whereby post-vaccination titers correlated negatively with age in the 0-1-3 scheme. Thus, the 0-1-6 scheme should continue to be the preferred HBV vaccination schedule, but interventions to improve compliance with the third vaccine dose are needed.
Keywords: Central Asia; Kyrgyzstan; aging; compliance; hepatitis B; immune response; study retention; vaccination; vaccine response.
Conflict of interest statement
Janyn Heisig was employed by the Serum Life Sciences Europe GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- World Health Organization . Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections, 2021: Accountability for the Global Health Sector Strategies 2016–2021: Actions for Impact. World Health Organization; Geneva, Switzerland: 2021. [(accessed on 4 December 2024)]. Available online: https://iris.who.int/handle/10665/341412.
-
- World Health Organization Fact-Sheet: Hepatitis. 2022. [(accessed on 14 April 2024)]. Available online: https://www.who.int/health-topics/hepatitis#tab=tab_1.
-
- Sheena B.S., Hiebert L., Han H., Ippolito H., Abbasi-Kangevari M., Abbasi-Kangevari Z., Abbastabar H., Abdoli A., Abubaker Ali H., Adane M.M., et al. Global, regional, and national burden of hepatitis B, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 2022;7:796–829. doi: 10.1016/S2468-1253(22)00124-8. - DOI - PMC - PubMed
-
- Preventing Perinatal Hepatitis B Virus Transmission: A Guide for Introducing and Strengthening Hepatitis B Birth Dose Vaccination. World Health Organization. 2015. [(accessed on 19 April 2024)]. Available online: https://apps.who.int/iris/bitstream/handle/10665/208278/9789241509831_en....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

