Effects of the Tobacco Defensin NaD1 Against Susceptible and Resistant Strains of Candida albicans
- PMID: 39770352
- PMCID: PMC11678012
- DOI: 10.3390/pathogens13121092
Effects of the Tobacco Defensin NaD1 Against Susceptible and Resistant Strains of Candida albicans
Abstract
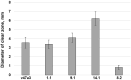
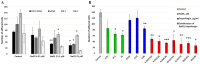
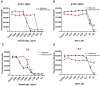
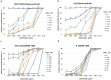
Today, Candida albicans is still the most common cause of both local and life-threatening systemic candidiasis. The spread of resistant fungal strains has resulted in an urgent need to search for new promising antimycotics. Here, we investigated the antifungal action of the tobacco defensin NaD1 against susceptible and resistant to azoles and echinocandins strains of C. albicans. We demonstrated that NaD1 was equally effective and fungicidal against all tested strains. The MIC and MFC values were 6.25 and 12.5 µM, respectively. We showed for the first time that NaD1 could act synergistically not only with caspofungin but also with human host defense antimicrobial peptides cathelicidin LL-37 and β-defensin-2 (HBD2) against susceptible and resistant fungal strains. Using flow cytometry, we demonstrated that NaD1 in combinations with LL-37 or HBD2 can reinforce each other by enhancing membrane disruption. Using the Caco-2 cell monolayer model, we demonstrated that NaD1 impaired the adhesion of C. albicans cells to the human epithelium. Moreover, NaD1 inhibited the formation of fungal biofilms in Sabouraud broth and less markedly in nutrient-rich RPMI-1640 medium, and enhanced the antibiofilm activity of caspofungin. Thus, we hypothesized that NaD1 might affect the development of candidiasis in vivo, including that caused by resistant fungal strains.
Keywords: Candida albicans; adherence; biofilms; candidiasis; caspofungin; clinical isolates; human cathelicidin LL-37; human β-defensin 2 (HBD2); membrane-disrupting activity; plant defensins; resistance; synergism; tobacco NaD1.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- World Health Organization WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. [(accessed on 28 November 2024)]. Available online: https://www.who.int/publications/i/item/9789240060241.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

