Modelling of Cetylpyridinium Chloride Availability in Complex Mixtures for the Prediction of Anti-Microbial Activity Using Diffusion Ordered Spectroscopy, Saturation Transfer Difference and 1D NMR
- PMID: 39770413
- PMCID: PMC11679348
- DOI: 10.3390/ph17121570
Modelling of Cetylpyridinium Chloride Availability in Complex Mixtures for the Prediction of Anti-Microbial Activity Using Diffusion Ordered Spectroscopy, Saturation Transfer Difference and 1D NMR
Abstract
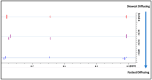
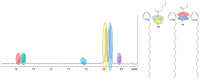
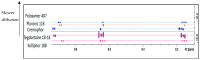
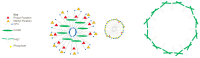
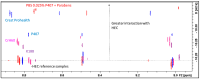
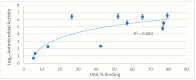
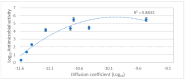
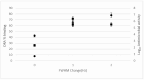
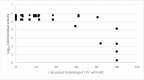
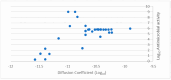
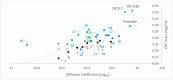
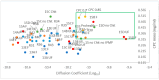
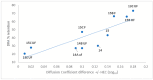
Background/Objectives: A range of NMR techniques, including diffusion ordered spectroscopy (DOSY) were used to characterise complex micelles formed by the anti-microbial cationic surfactant cetylpyridium chloride and to quantify the degree of interaction between cetylpyridium chloride and hydroxyethyl cellulose in a variety of commercially relevant formulations as a model for the disk retention assay. Methods: This NMR-derived binding information was then compared with the results of formulation analysis by traditional disk retention assay (DRA) and anti-microbial activity assays to assess the suitability of these NMR techniques for the rapid identification of formulation components that could augment or retard antimicrobial activity DRA. Results: NMR showed a strong ability to predict anti-microbial activity for a diverse range of formulations containing cetylpyridinium chloride (CPC). Conclusions: This demonstrates the value of this NMR-based approach as a rapid, relatively non-destructive method for screening commercial experimental anti-microbial formulations for efficacy and further helps to understand the interplay of excipients and active ingredients.
Keywords: NMR; anti-microbial; cetylpyridinium chloride; diffusion; formulation; micelles.
Conflict of interest statement
Author Sayoni Batabyal, Darren Whitworth, Angharad Smith, Alessandra Montesanto, and Robert Lucas were employed by the company Haleon. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Tomris and Cameron were funded by Haleon, but analysis and evaluation was carried out independent of Haleon PLC.
Figures


Similar articles
-
Evaluation of Cetylpyridinium Chloride-Containing Mouthwashes Using In Vitro Disk Retention and Ex Vivo Plaque Glycolysis Methods.J Clin Dent. 1997;8(4):107-13. J Clin Dent. 1997. PMID: 26630720
-
Comparative bioavailability and antimicrobial activity of cetylpyridinium chloride mouthrinses in vitro and in vivo.Am J Dent. 2014 Aug;27(4):185-90. Am J Dent. 2014. PMID: 25831600 Clinical Trial.
-
Rechargeable anti-microbial adhesive formulation containing cetylpyridinium chloride montmorillonite.Acta Biomater. 2019 Dec;100:388-397. doi: 10.1016/j.actbio.2019.09.045. Epub 2019 Sep 27. Acta Biomater. 2019. PMID: 31568874
-
Excipient interaction with cetylpyridinium chloride activity in tablet based lozenges.Pharm Res. 1996 Aug;13(8):1258-64. doi: 10.1023/a:1016084824877. Pharm Res. 1996. PMID: 8865323
-
Pulsed-field gradient nuclear magnetic resonance measurements (PFG NMR) for diffusion ordered spectroscopy (DOSY) mapping.Analyst. 2017 Oct 9;142(20):3771-3796. doi: 10.1039/c7an01031a. Analyst. 2017. PMID: 28858342 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

