Bilosomes and Niosomes for Enhanced Intestinal Absorption and In Vivo Efficacy of Cytarabine in Treatment of Acute Myeloid Leukemia
- PMID: 39770414
- PMCID: PMC11677554
- DOI: 10.3390/ph17121572
Bilosomes and Niosomes for Enhanced Intestinal Absorption and In Vivo Efficacy of Cytarabine in Treatment of Acute Myeloid Leukemia
Abstract
Cytarabine (CTR) is a hydrophilic anticancer drug used to treat leukemia. It suffers from poor permeability and intestinal metabolism, diminishing its oral bioavailability.
Background/objectives: The objective was to develop and evaluate niosomes and bilosomes for enhanced intestinal absorption; hence, oral bioavailability.
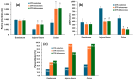
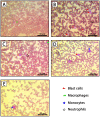
Results: CTR-loaded niosomes and bilosomes with vesicle sizes of 152 and 204.3 nm were successfully prepared with acceptable properties. The presence of bile salts increased the zeta potential of bilosomes. The recorded entrapment efficiency of cytarabine was acceptable for such a hydrophilic drug. CTR-bilosomes showed a pH-dependent drug release pattern with preferred release in pH 6.8. Intestinal absorption behavior indicated a site-dependent CTR absorption pattern with unfavorable absorption in the distal intestine. Niosomal and bilosomal formulations enhanced intestinal absorption parameters with evidence for a predominant paracellular absorption mechanism that bypasses intestinal barriers. The investigation of the anti-leukemic effect of niosomal and bilosomal formulations indicated that both formulations ameliorated the blood parameters, reflecting significant improvement in leukemia treatment compared with the drug solution. Pathological examination of blood films revealed decreased blast cells in peripheral blood in groups treated with tested formulations.
Methods: Tested formulations were prepared according to the pro-concentrate method and characterized for particle size, zeta potential, entrapment efficiency, and in vitro release. CTR-loaded niosomes and bilosomes were evaluated for enhanced intestinal absorption utilizing the single-pass in situ intestinal perfusion method in rabbits, and the anti-leukemic effect was assessed using the benzene-induced leukemia model in rats.
Conclusions: This study introduced surfactant vesicles for enhanced oral bioavailability of CTR.
Keywords: bilosomes; cytarabine; intestinal perfusion; leukemia; niosomes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Niosomal carriers enhance oral bioavailability of carvedilol: effects of bile salt-enriched vesicles and carrier surface charge.Int J Nanomedicine. 2015 Jul 29;10:4797-813. doi: 10.2147/IJN.S84703. eCollection 2015. Int J Nanomedicine. 2015. PMID: 26251598 Free PMC article.
-
Permeation enhancers loaded bilosomes for improved intestinal absorption and cytotoxic activity of doxorubicin.Int J Pharm. 2023 Jan 5;630:122427. doi: 10.1016/j.ijpharm.2022.122427. Epub 2022 Nov 23. Int J Pharm. 2023. PMID: 36435504
-
Niosomes for oral delivery of nateglinide: in situ-in vivo correlation.J Liposome Res. 2018 Sep;28(3):209-217. doi: 10.1080/08982104.2017.1343835. Epub 2017 Jul 2. J Liposome Res. 2018. PMID: 28618876
-
Bilosomes as Nanocarriers for the Drug and Vaccine Delivery against Gastrointestinal Infections: Opportunities and Challenges.J Funct Biomater. 2023 Sep 1;14(9):453. doi: 10.3390/jfb14090453. J Funct Biomater. 2023. PMID: 37754867 Free PMC article. Review.
-
The Pharmaceutical and Pharmacological Potential Applications of Bilosomes as Nanocarriers for Drug Delivery.Molecules. 2025 Mar 6;30(5):1181. doi: 10.3390/molecules30051181. Molecules. 2025. PMID: 40076403 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

