Ocular and Plasma Pharmacokinetics of Sitagliptin Eye Drops: Preclinical Data
- PMID: 39770421
- PMCID: PMC11676928
- DOI: 10.3390/ph17121579
Ocular and Plasma Pharmacokinetics of Sitagliptin Eye Drops: Preclinical Data
Abstract
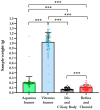
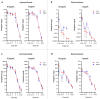
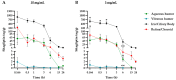
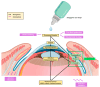
Background/Objectives: Early stages of diabetic retinopathy are currently considered an unmet medical need due to the lack of effective treatments beyond proper monitoring and control of glycemia and blood pressure. Sitagliptin eye drops have emerged as a new therapeutic approach against early stages of the disease, as they can prevent its main hallmarks, including both neurodegeneration and microvascular impairment. Interestingly, all of these effects occur without any glycemic systemic improvement. In the present study, we aimed to investigate the pharmacokinetics and distribution of the drug within the eye and plasma. Methods: A total of 48 male New Zealand rabbits were treated with topical administration (eye drops) of sitagliptin at two concentrations: 5 mg/mL and 10 mg/mL. Blood, iris/ciliary body, retina/choroid, aqueous humor, and vitreous humor samples were collected at specific intervals post-administration (10 and 30 min and 1, 3, 6, 15, and 24 h), processed, and analyzed using an LC-MS/MS method. The pharmacokinetics of sitagliptin were then calculated, and statistical comparisons were performed. Results: Our findings indicate that sitagliptin reaches the retina prior to the aqueous and vitreous humors, suggesting that its absorption follows the transscleral route. Additionally, systemic absorption was minimal and below pharmacologically active concentrations. Conclusions: These results support the use of an eye drop formulation for the treatment of diabetic retinopathy and other retinal diseases.
Keywords: diabetic retinopathy; dipeptidyl peptidase-4 inhibitor; eye drops; pharmacokinetics; sitagliptin; transscleral.
Conflict of interest statement
Two of the authors (Cristina Hernández and Rafael Simó) are inventors of the patent PCT/EP2017/060234 (see above).
Figures

Similar articles
-
Sitagliptin eye drops prevent the impairment of retinal neurovascular unit in the new Trpv2+/- rat model.J Neuroinflammation. 2024 Nov 30;21(1):312. doi: 10.1186/s12974-024-03283-5. J Neuroinflammation. 2024. PMID: 39616390 Free PMC article.
-
Ocular Biodistribution of Once-Daily 0.6% Bilastine Eye Drops Reveals Highest Levels in Conjunctiva Up to 24 h Postadministration.J Ocul Pharmacol Ther. 2022 Nov;38(9):617-625. doi: 10.1089/jop.2022.0024. Epub 2022 Oct 21. J Ocul Pharmacol Ther. 2022. PMID: 36269652 Free PMC article.
-
Bio-Distribution and Pharmacokinetics of Topically Administered γ-Cyclodextrin Based Eye Drops in Rabbits.Pharmaceuticals (Basel). 2021 May 18;14(5):480. doi: 10.3390/ph14050480. Pharmaceuticals (Basel). 2021. PMID: 34070168 Free PMC article.
-
Ocular pharmacokinetics of naringenin eye drops following topical administration to rabbits.J Ocul Pharmacol Ther. 2015 Feb;31(1):51-6. doi: 10.1089/jop.2014.0047. J Ocul Pharmacol Ther. 2015. PMID: 25229266 Free PMC article.
-
Transcriptomic Analysis Reveals That Retinal Neuromodulation Is a Relevant Mechanism in the Neuroprotective Effect of Sitagliptin in an Experimental Model of Diabetic Retinopathy.Int J Mol Sci. 2022 Dec 29;24(1):571. doi: 10.3390/ijms24010571. Int J Mol Sci. 2022. PMID: 36614016 Free PMC article.
References
-
- Gonzalez-Cortes J.H., Martinez-Pacheco V.A., Gonzalez-Cantu J.E., Bilgic A., de Ribot F.M., Sudhalkar A., Mohamed-Hamsho J., Kodjikian L., Mathis T. Current Treatments and Innovations in Diabetic Retinopathy and Diabetic Macular Edema. Pharmaceutics. 2022;15:122. doi: 10.3390/pharmaceutics15010122. - DOI - PMC - PubMed
-
- Hernández C., Bogdanov P., Corraliza L., García-Ramírez M., Solà-Adell C., Arranz J.A., Arroba A.I., Valverde A.M., Simó R. Topical Administration of GLP-1 Receptor Agonists Prevents Retinal Neurodegeneration in Experimental Diabetes. Diabetes. 2016;65:172–187. doi: 10.2337/db15-0443. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

