Adjuvant Effect of Lactobacillus paracasei in Sublingual Immunotherapy of Asthmatic Mice
- PMID: 39770422
- PMCID: PMC11678203
- DOI: 10.3390/ph17121580
Adjuvant Effect of Lactobacillus paracasei in Sublingual Immunotherapy of Asthmatic Mice
Abstract
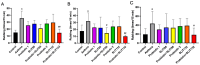
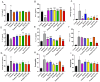
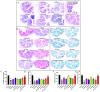
Background: Sublingual immunotherapy (SLIT) has shown promise in mitigating allergic asthma symptoms; nevertheless, its high dose and prolonged duration of treatment raise safety concerns. This study explored the potential of Lactobacillus paracasei (L. paracasei) to enhance the effectiveness of SLIT in a mouse model of allergic asthma. Methods: Allergic asthma was induced in Balb/c mice following sensitization and challenge with a house dust mite (HDM) allergen. Subsequently, the mice were subjected to SLIT (66 and 132 µg) either alone or in combination with L. paracasei supplementation. Asthma-associated parameters, including rubbing frequency, IgE level, cytokine profiles, and histological changes, were evaluated to assess treatment efficacy. Results: mice that received SLIT 132 µg combined with the probiotic (combined 132) demonstrated a significant reduction in allergic symptoms (rubbing). This treatment strategy led to a marked IgE and eosinophil level decrease in serum; an increase in anti-inflammatory cytokines like IFN-γ and IL-10; and a reduction in pro-inflammatory cytokines IL-17 and TNF-α. The combination therapy also mitigated lung inflammation and supported the restoration of the structural integrity of the colon, promoting the recovery of goblet cells and mucus secretion. Probiotic treatment alone also effectively reduced IgE levels, increased IFN-γ, and decreased levels of IL-17 and TNF-α. Conclusions: The adjuvant effect of L. paracasei in enhancing SLIT represents a promising approach for improving asthma treatment efficacy.
Keywords: allergic asthma; house dust mite; probiotic; sublingual immunotherapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Guideline on allergen-specific immunotherapy in IgE-mediated allergic diseases: S2k Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Society for Pediatric Allergy and Environmental Medicine (GPA), the Medical Association of German Allergologists (AeDA), the Austrian Society for Allergy and Immunology (ÖGAI), the Swiss Society for Allergy and Immunology (SGAI), the German Society of Dermatology (DDG), the German Society of Oto- Rhino-Laryngology, Head and Neck Surgery (DGHNO-KHC), the German Society of Pediatrics and Adolescent Medicine (DGKJ), the Society for Pediatric Pneumology (GPP), the German Respiratory Society (DGP), the German Association of ENT Surgeons (BV-HNO), the Professional Federation of Paediatricians and Youth Doctors (BVKJ), the Federal Association of Pulmonologists (BDP) and the German Dermatologists Association (BVDD).Allergo J Int. 2014;23(8):282-319. doi: 10.1007/s40629-014-0032-2. Allergo J Int. 2014. PMID: 26120539 Free PMC article.
-
Methods for Experimental Allergen Immunotherapy: Subcutaneous and Sublingual Desensitization in Mouse Models of Allergic Asthma.Methods Mol Biol. 2021;2223:295-335. doi: 10.1007/978-1-0716-1001-5_20. Methods Mol Biol. 2021. PMID: 33226602
-
Subcutaneous and Sublingual Immunotherapy in a Mouse Model of Allergic Asthma.Methods Mol Biol. 2017;1559:137-168. doi: 10.1007/978-1-4939-6786-5_11. Methods Mol Biol. 2017. PMID: 28063043
-
Efficacy and Safety of House Dust Mite Sublingual Immunotherapy Tablet in Allergic Asthma: A Systematic Review of Randomized Controlled Trials.J Allergy Clin Immunol Pract. 2022 May;10(5):1342-1355.e24. doi: 10.1016/j.jaip.2022.01.046. Epub 2022 Feb 15. J Allergy Clin Immunol Pract. 2022. PMID: 35181547
-
An evidence-based analysis of house dust mite allergen immunotherapy: a call for more rigorous clinical studies.J Allergy Clin Immunol. 2013 Dec;132(6):1322-36. doi: 10.1016/j.jaci.2013.09.004. Epub 2013 Oct 18. J Allergy Clin Immunol. 2013. PMID: 24139829 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

