Study of Cytotoxicity of Spiro-Fused [3-Azabicyclo[3.1.0]hexane]oxindoles and Cyclopropa[a]pyrrolizidine-oxindoles Against Tumor Cell Lines
- PMID: 39770424
- PMCID: PMC11680018
- DOI: 10.3390/ph17121582
Study of Cytotoxicity of Spiro-Fused [3-Azabicyclo[3.1.0]hexane]oxindoles and Cyclopropa[a]pyrrolizidine-oxindoles Against Tumor Cell Lines
Abstract
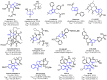
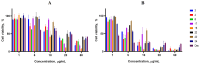
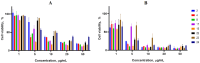
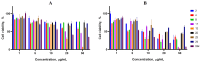
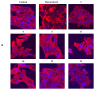
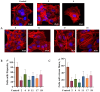
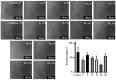
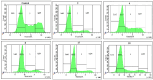
Background: A series of spiro-fused heterocyclic compounds containing cyclopropa[a]pyrrolizidine-2,3'-oxindole and 3-spiro[3-azabicyclo[3.1.0]-hexane]oxindole frameworks were synthesized and studied for their in vitro antiproliferative activity against human erythroleukemia (K562), cervical carcinoma (HeLa), acute T cell leukemia (Jurkat), melanoma (Sk-mel-2) and breast cancer (MCF-7) as well as mouse colon carcinoma (CT26) cell lines. Methods: Cell proliferation was evaluated in vitro by MTS assay. Confocal microscopy was used to study actin cytoskeleton structure and cell motility. Cell cycle analysis was evaluated by flow cytometry. Results: It was found that compounds 4, 8, 18 and 24 showed antiproliferative activity against the Jurkat, K-562, HeLa and Sk-mel-2 cell lines with IC50 ranging from 2 to 10 μM (72 h). Evaluation of the impact on cell cycle progression showed that the tested compounds achieved significant cell-cycle perturbation with a higher accumulation of cells in the SubG1 and G0/G1 phases of the cell cycle, in comparison to the negative control. I Incubation with tested compounds led to the disappearance of stress fibers (granular actin was distributed diffusely in the cytoplasm in up to 38% of treated HeLa cells) and changes in the number of filopodia-like deformations (reduced from 93% in control cells to 64% after treatment). The impact on the Sk-mel-2 cell actin cytoskeleton structure was even greater: granular actin was distributed diffusely in the cytoplasm in up to 90% of treated cells while the number of filopodia-like deformations was reduced by up to 23%. A scratch test performed on the human melanoma cell line showed that these cells did not fill the scratched strip and lose their ability to move under treatment. Conclusions: The obtained results support the antitumor effect of the tested spiro-compounds and encourage the extension of this study in order to improve their anticancer activity as well as reduce their toxicological risks.
Keywords: 1,3-dipolar cycloaddition; 3-spiro[3-azabicyclo[3.1.0]-hexane]oxindole; antiproliferative activity; azomethine ylides; cell cycle; cell motility; cyclopropa[a]pyrrolizidine-2,3′-oxindole; cyclopropenes; morphological changes (cytoskeleton); tumor cell lines.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

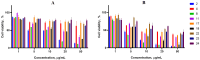


References
-
- Lahlou M. The Success of Natural Products in Drug Discovery. Pharmacol. Pharm. 2013;4:17–31. doi: 10.4236/pp.2013.43A003. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

