Theoretical-Cheminformatic Study of Four Indolylphytoquinones, Prospective Anticancer Candidates
- PMID: 39770437
- PMCID: PMC11679286
- DOI: 10.3390/ph17121595
Theoretical-Cheminformatic Study of Four Indolylphytoquinones, Prospective Anticancer Candidates
Abstract
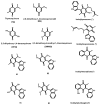
Background/Objectives: Breast cancer is a disease with a high mortality rate worldwide; consequently, urgent achievements are required to design new greener drugs, leaving natural products and their derivatives as good options. A constant antineoplastic effect has been observed when the phytoproduct contains an indole fragment. Methods: Therefore, the objective of this work was to carry out a thoughtful computational study to perform an appropriate evaluation of four novel molecules of the class of the 3-indolylquinones as phytodrug candidates for antineoplastic activity: thymoquinone (TQ), 2,6-dimethoxy-1,4-benzoquinone (DMQ), 2,3-dimethoxy-5-methyl-1,4-benzoquinone (DMMQ), and 2,5-dihydroxy-1,4-benzoquinone (DHQ). It is important to highlight that the obtained computational results of the target compounds were compared-correlated with the theoretical and experimental literature data previously reported of several indolylquinones: indolylperezone, indolylisoperezone, indolylmenadione, and indolylplumbagin (IE-IH, respectively). Results: The results revealed that the studied structures possibly presented antineoplastic activity, in addition to the fact that the reactivity parameters showed that two of the evaluated compounds have the option to present IC50 values lower than or similar to 25 mg/mL, activity like that of indolylisoperezone; moreover, they show molecular coupling to PARP-1. Finally, the prediction of the calculated physicochemical parameters coincides with the Lipinski and Veber rules, indicating that the adsorption, metabolism, and toxicity parameters are acceptable for the studied compounds, obtaining high drug score values. Conclusions: Finally, a comparison between the proposed molecules and others previously synthesized was appropriately performed, establishing that the synthesis of the studied compounds and the determination of their pharmacological properties in an experimental manner are of interest.
Keywords: DFT; chemoinformatic tools; in silico study; indolylquinones; molecular docking.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









References
-
- Ariza Márquez Y.V., Briceño Balcázar I., Ancízar Aristizábal F. Tratamiento de Cáncer de Seno y Farmacogenética. Rev. Colomb. Biotecnol. 2016;18:121–134. doi: 10.15446/rev.colomb.biote.v18n1.57723. - DOI
-
- Argüello Esparza E.Y. Cáncer de la Mujer. Centro Nacional de Equidad de Género y Salud Reproductiva; Mexico City, Mexico: 2015.
-
- Instituto Nacional de Estadística y Geografía . Estadísticas a Propósito del día Internacional de la Lucha Contra el Cáncer de Mama (19 de Octubre) Instituto Nacional de Estadística y Geografía; Mexico City, Mexico: 2023.
LinkOut - more resources
Full Text Sources
Miscellaneous

