Suppression of NNK Metabolism by Anthocyanin-Rich Haskap Berry Supplementation Through Modulation of P450 Enzymes
- PMID: 39770457
- PMCID: PMC11728747
- DOI: 10.3390/ph17121615
Suppression of NNK Metabolism by Anthocyanin-Rich Haskap Berry Supplementation Through Modulation of P450 Enzymes
Abstract
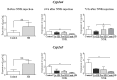
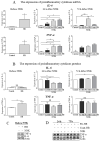
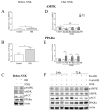
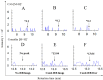
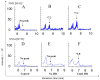
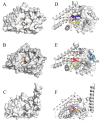
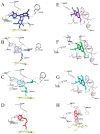
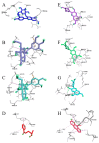
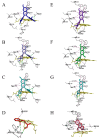
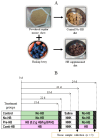
Oral supplementation of anthocyanins-rich haskap (Lonicera caerulea) berry (HB) reduces 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)-induced lung tumorigenesis, cytotoxicity, DNA damage, and modulated inflammation in vitro and in vivo. The procarcinogen NNK is metabolically activated by cytochrome P450 (P450) enzymes, producing reactive metabolites that induce lung carcinogenesis. Hypothesis: Therefore, we hypothesized that the HB-modulated protective effect against NNK could be due to its ability to suppress P450 enzymes. Methods: HB (6 mg of cyanidin-3-O-glucoside [C3G] in 0.2 g of HB/mouse/day) was given to A/J mice as a dietary supplement following subsequent administration of NNK (100 mg/kg body weight). The liver tissues of mice were analyzed to determine the expression of P450s and metabolites. Results: HB upregulated the expression of cyp2a4 and cyp2a5 mRNA and nuclear receptor/transcription factor (PPARα) in NNK-deprived hepatic tissues. With NNK, HB downregulated the expression of cyp2a4 and cyp2a5 and facilitated the formation of non-carcinogenic NNK metabolites. Molecular docking indicated a high binding affinity and strong hydrophobic interactions between C3G and its major metabolites, peonidin-3-O-glucoside, petunidin-3-O-glucoside, peonidin and cyanidin with Cyp2a5 and with human P450 homologue CYP2A13. Conclusions: HB could be a potential dietary supplement to inhibit the P450 activated NNK carcinogenic metabolites formation. Hence, inhibiting the activation of NNK by lung CYP2A13 through dietary HB supplementation could be a strategy to reduce lung carcinogenesis among smokers. Understanding the effect of HB on the activity of CYP2A13 in human studies is necessary before recommending these natural compounds as therapeutics.
Keywords: blue honeysuckle; carcinogen; cytochrome P450; hepatic metabolism; lung cancer; smoking.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in designing the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Cyanidin-3-O-Glucoside-Rich Haskap Berry Administration Suppresses Carcinogen-Induced Lung Tumorigenesis in A/JCr Mice.Molecules. 2020 Aug 22;25(17):3823. doi: 10.3390/molecules25173823. Molecules. 2020. PMID: 32842605 Free PMC article.
-
Role of CYP2A13 in the bioactivation and lung tumorigenicity of the tobacco-specific lung procarcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone: in vivo studies using a CYP2A13-humanized mouse model.Carcinogenesis. 2014 Jan;35(1):131-7. doi: 10.1093/carcin/bgt269. Epub 2013 Aug 5. Carcinogenesis. 2014. PMID: 23917075 Free PMC article.
-
Anthocyanin-rich haskap (Lonicera caerulea L.) berry extracts reduce nitrosamine-induced DNA damage in human normal lung epithelial cells in vitro.Food Chem Toxicol. 2020 Jul;141:111404. doi: 10.1016/j.fct.2020.111404. Epub 2020 May 13. Food Chem Toxicol. 2020. PMID: 32413456
-
Identification of cytochrome P450 enzymes critical for lung tumorigenesis by the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK): insights from a novel Cyp2abfgs-null mouse.Carcinogenesis. 2014 Nov;35(11):2584-91. doi: 10.1093/carcin/bgu182. Epub 2014 Aug 30. Carcinogenesis. 2014. PMID: 25173884 Free PMC article.
-
Recent studies on mechanisms of bioactivation and detoxification of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), a tobacco-specific lung carcinogen.Crit Rev Toxicol. 1996;26(2):163-81. doi: 10.3109/10408449609017929. Crit Rev Toxicol. 1996. PMID: 8688159 Review.
Cited by
-
Development of a Scalable Extraction Process for Anthocyanins of Haskap Berry (Lonicera caerulea).Molecules. 2025 Feb 26;30(5):1071. doi: 10.3390/molecules30051071. Molecules. 2025. PMID: 40076296 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

