N-methyl-D-aspartate Receptors and Depression: Linking Psychopharmacology, Pathology and Physiology in a Unifying Hypothesis for the Epigenetic Code of Neural Plasticity
- PMID: 39770460
- PMCID: PMC11728621
- DOI: 10.3390/ph17121618
N-methyl-D-aspartate Receptors and Depression: Linking Psychopharmacology, Pathology and Physiology in a Unifying Hypothesis for the Epigenetic Code of Neural Plasticity
Abstract
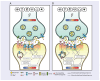
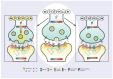
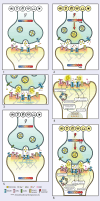
Uncompetitive NMDAR (N-methyl-D-aspartate receptor) antagonists restore impaired neural plasticity, reverse depressive-like behavior in animal models, and relieve major depressive disorder (MDD) in humans. This review integrates recent findings from in silico, in vitro, in vivo, and human studies of uncompetitive NMDAR antagonists into the extensive body of knowledge on NMDARs and neural plasticity. Uncompetitive NMDAR antagonists are activity-dependent channel blockers that preferentially target hyperactive GluN2D subtypes because these subtypes are most sensitive to activation by low concentrations of extracellular glutamate and are more likely activated by certain pathological agonists and allosteric modulators. Hyperactivity of GluN2D subtypes in specific neural circuits may underlie the pathophysiology of MDD. We hypothesize that neural plasticity is epigenetically regulated by precise Ca2+ quanta entering cells via NMDARs. Stimuli reach receptor cells (specialized cells that detect specific types of stimuli and convert them into electrical signals) and change their membrane potential, regulating glutamate release in the synaptic cleft. Free glutamate binds ionotropic glutamatergic receptors regulating NMDAR-mediated Ca2+ influx. Quanta of Ca2+ via NMDARs activate enzymatic pathways, epigenetically regulating synaptic protein homeostasis and synaptic receptor expression; thereby, Ca2+ quanta via NMDARs control the balance between long-term potentiation and long-term depression. This NMDAR Ca2+ quantal hypothesis for the epigenetic code of neural plasticity integrates recent psychopharmacology findings into established physiological and pathological mechanisms of brain function.
Keywords: AMPA; Ca2+; Mg2+; NMDA; endorphins; glutamate; major depressive disorder; neural plasticity.
Conflict of interest statement
The authors declare that this work received funding from Relmada Therapeutics, Inc. The funder had no involvement with this work. S.C., S.D.M., A.M., C.G., M.P., F.F., A.A. and P.L.M. are employed by or have received compensation from from Relmada Therapeutics, Inc or from companies or institutions that received funding from Relmada Therapeutics, Inc. P.L.M. is inventor of technology related to esmethadone.
Figures
Similar articles
-
Esmethadone (REL-1017) and Other Uncompetitive NMDAR Channel Blockers May Improve Mood Disorders via Modulation of Synaptic Kinase-Mediated Signaling.Int J Mol Sci. 2022 Oct 13;23(20):12196. doi: 10.3390/ijms232012196. Int J Mol Sci. 2022. PMID: 36293063 Free PMC article.
-
Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors.Crit Rev Neurobiol. 2006;18(1-2):71-84. doi: 10.1615/critrevneurobiol.v18.i1-2.80. Crit Rev Neurobiol. 2006. PMID: 17725510 Review.
-
BNST GluN2D-Containing NMDA Receptors Influence Anxiety- and Depressive-like Behaviors and ModulateCell-Specific Excitatory/Inhibitory Synaptic Balance.J Neurosci. 2020 May 13;40(20):3949-3968. doi: 10.1523/JNEUROSCI.0270-20.2020. Epub 2020 Apr 10. J Neurosci. 2020. PMID: 32277042 Free PMC article.
-
Non-Ionotropic NMDA Receptor Signaling Drives Activity-Induced Dendritic Spine Shrinkage.J Neurosci. 2015 Sep 2;35(35):12303-8. doi: 10.1523/JNEUROSCI.4289-14.2015. J Neurosci. 2015. PMID: 26338340 Free PMC article.
-
Allosteric modulators of NR2B-containing NMDA receptors: molecular mechanisms and therapeutic potential.Br J Pharmacol. 2009 Aug;157(8):1301-17. doi: 10.1111/j.1476-5381.2009.00304.x. Epub 2009 Jul 8. Br J Pharmacol. 2009. PMID: 19594762 Free PMC article. Review.
Cited by
-
Glycogen synthase kinase-3: the master switch driving neurodegeneration in Alzheimer's disease and Parkinson's disease.Arch Toxicol. 2025 Aug 28. doi: 10.1007/s00204-025-04174-1. Online ahead of print. Arch Toxicol. 2025. PMID: 40866602 Review.
-
[Prefrontal dysfunction and mismatch negativity in adolescent depression: A multimodal fNIRS-ERP study].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2025 Aug 25;42(4):701-706. doi: 10.7507/1001-5515.202503053. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2025. PMID: 40887184 Free PMC article. Chinese.
-
New Synthesis and Pharmacological Evaluation of Enantiomerically Pure (R)- and (S)-Methadone Metabolites as N-Methyl-d-aspartate Receptor Antagonists.J Med Chem. 2025 Mar 13;68(5):5455-5470. doi: 10.1021/acs.jmedchem.4c02605. Epub 2025 Feb 25. J Med Chem. 2025. PMID: 39999356 Free PMC article.
References
-
- Balestrieri M., Rucci P., Amendola D., Bonizzoni M., Cerveri G., Colli C., Dragogna F., Ducci G., Elmo M.G., Ghio L., et al. Emergency Psychiatric Consultations During and After the COVID-19 Lockdown in Italy. A Multicentre Study. Front. Psychiatry. 2021;12:697058. doi: 10.3389/fpsyt.2021.697058. - DOI - PMC - PubMed
-
- American Psychiatric Association . DSM 5. American Psychiatric Association; Washington, DC, USA: 2013.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

