Biopolymeric Inhalable Dry Powders for Pulmonary Drug Delivery
- PMID: 39770469
- PMCID: PMC11728674
- DOI: 10.3390/ph17121628
Biopolymeric Inhalable Dry Powders for Pulmonary Drug Delivery
Abstract
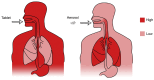
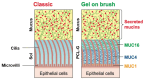
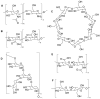
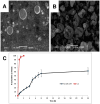
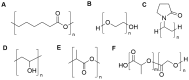
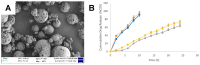
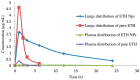
Natural and synthetic biopolymers are gaining popularity in the development of inhaled drug formulations. Their highly tunable properties and ability to sustain drug release allow for the incorporation of attributes not achieved in dry powder inhaler formulations composed only of micronized drugs, standard excipients, and/or carriers. There are multiple physiological barriers to the penetration of inhaled drugs to the epithelial surface, such as the periciliary layer mucus mesh, pulmonary macrophages, and inflammation and mucus compositional changes resulting from respiratory diseases. Biopolymers may facilitate transport to the epithelial surface despite such barriers. A variety of categories of biopolymers have been assessed for their potential in inhaled drug formulations throughout the research literature, ranging from natural biopolymers (e.g., chitosan, alginate, hyaluronic acid) to those synthesized in a laboratory setting (e.g., polycaprolactone, poly(lactic-co-glycolic acid)) with varying structures and compositions. To date, no biopolymers have been approved as a commercial dry powder inhaler product. However, advances may be possible in the treatment of respiratory diseases and infections upon further investigation and evaluation. Herein, this review will provide a thorough foundation of reported research utilizing biopolymers in dry powder inhaler formulations. Furthermore, insight and considerations for the future development of dry powder formulations will be proposed.
Keywords: biopolymers; dry powder inhalers; poly(lactic-co-glycolic acid); polycaprolactone; polysaccharides.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Hickey A.J., Thompson D.C. Physiology of the Airways. In: Hickey A.J., editor. Pharmaceutical Inhalation Aerosol Technology. Marcel Dekker, Inc.; New York, NY, USA: 2004. pp. 1–29.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

