The Melatonin Type 2 Receptor Agonist IIK7 Attenuates and Reverses Morphine Tolerance in Neuropathic Pain Rats Through the Suppression of Neuroinflammation in the Spinal Cord
- PMID: 39770480
- PMCID: PMC11676719
- DOI: 10.3390/ph17121638
The Melatonin Type 2 Receptor Agonist IIK7 Attenuates and Reverses Morphine Tolerance in Neuropathic Pain Rats Through the Suppression of Neuroinflammation in the Spinal Cord
Abstract
Background: Morphine analgesic tolerance (MAT) limits the clinical application of morphine in the management of chronic pain. IIK7 is a melatonin type 2 (MT2) receptor agonist known to have antioxidant properties. Oxidative stress is recognized as a critical factor in MAT. This study sought to assess the impact of IIK7 on the progression of MAT and its potential to reverse pre-existing MAT.
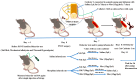
Methods: Wistar rats underwent partial sciatic nerve transection (PSNT) surgery to induce neuropathic pain (NP). Seven days post nerve transection, we implanted an intrathecal (i.t.) catheter and linked it to an osmotic pump. Rats were randomly divided into the following groups: sham-operated/vehicle, PSNT/vehicle, PSNT/IIK7 50 ng/h, PSNT/MOR 15 g/h, and PSNT/MOR 15 g + IIK7 50 ng/h. We implanted two i.t. catheters for drug administration and the evaluation of the efficacy of IIK7 in reversing pre-established MAT. We linked one to an osmotic pump for MOR or saline continuous i.t. infusion. On the 7th day, the osmotic pump was disconnected, and 50 μg of IIK7 or the vehicle was administered through the second catheter. After 3 h, 15 μg of MOR or saline was administered, and the animal behavior tests were performed. We measured the levels of mRNA for Nrf2 and HO-1, pro-inflammatory cytokines (PICs), and the microglial and astrocyte activation in the spinal cord.
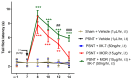
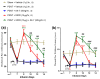
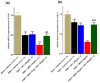
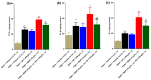
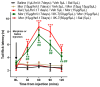
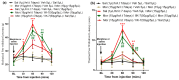
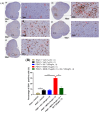
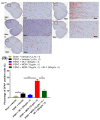
Results: The co-administration of IIK7 with MOR delayed MAT development in PSNT rats by restoring Nrf2 and HO-1 while also inhibiting the microglial-cell and astrocyte activation, alongside the suppression of PICs. Additionally, a single injection of high-dose 50 μg IIK7 was efficient in restoring MOR's antinociception in MOR-tolerant rats.
Conclusions: Our results indicate that the co-infusion of ultra-low-dose IIK7 can delay MAT development and a high dose can reverse pre-existing MAT.
Keywords: IIK7; MOR tolerance; MT2 receptor agonist; neuroinflammation; neuropathic pain.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Melatonin MT2 receptor agonist IIK-7 produces antinociception by modulation of ROS and suppression of spinal microglial activation in neuropathic pain rats.J Pain Res. 2019 Aug 8;12:2473-2485. doi: 10.2147/JPR.S214671. eCollection 2019. J Pain Res. 2019. PMID: 31496789 Free PMC article.
-
Teneligliptin Co-Infusion Alleviates Morphine Tolerance by Inhibition of Spinal Microglial Cell Activation in Streptozotocin-Induced Diabetic Rats.Antioxidants (Basel). 2023 Jul 24;12(7):1478. doi: 10.3390/antiox12071478. Antioxidants (Basel). 2023. PMID: 37508016 Free PMC article.
-
Circadian Light Manipulation and Melatonin Supplementation Enhance Morphine Antinociception in a Neuropathic Pain Rat Model.Int J Mol Sci. 2025 Jul 30;26(15):7372. doi: 10.3390/ijms26157372. Int J Mol Sci. 2025. PMID: 40806499 Free PMC article.
-
Upregulation of μ-Opioid Receptor in the Rat Spinal Cord Contributes to the α2-Adrenoceptor Agonist Dexmedetomidine-Induced Attenuation of Chronic Morphine Tolerance in Cancer Pain.J Pain Res. 2020 Oct 15;13:2617-2627. doi: 10.2147/JPR.S274225. eCollection 2020. J Pain Res. 2020. PMID: 33116804 Free PMC article.
-
Low-Dose Cannabinoid Type 2 Receptor Agonist Attenuates Tolerance to Repeated Morphine Administration via Regulating μ-Opioid Receptor Expression in Walker 256 Tumor-Bearing Rats.Anesth Analg. 2016 Apr;122(4):1031-7. doi: 10.1213/ANE.0000000000001129. Anesth Analg. 2016. PMID: 26720619
Cited by
-
Neuro-Nutritional Approach to Neuropathic Pain Management: A Critical Review.Nutrients. 2025 Apr 29;17(9):1502. doi: 10.3390/nu17091502. Nutrients. 2025. PMID: 40362812 Free PMC article. Review.
-
Comparative Analysis of Melatonin and Polydeoxyribonucleotide: Possible Benefits of Co-Treatment Effects and Potential Synergistic Applicability.Int J Mol Sci. 2025 Jun 13;26(12):5703. doi: 10.3390/ijms26125703. Int J Mol Sci. 2025. PMID: 40565167 Free PMC article. Review.
-
Spinal astrocytes involved in the pathogenesis and treatment of neuropathic pain.Front Cell Neurosci. 2025 Feb 21;19:1547524. doi: 10.3389/fncel.2025.1547524. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40062207 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

