An Azomethine Derivative, BCS3, Targets XIAP and cIAP1/2 to Arrest Breast Cancer Progression Through MDM2-p53 and Bcl-2-Caspase Signaling Modulation
- PMID: 39770487
- PMCID: PMC11678930
- DOI: 10.3390/ph17121645
An Azomethine Derivative, BCS3, Targets XIAP and cIAP1/2 to Arrest Breast Cancer Progression Through MDM2-p53 and Bcl-2-Caspase Signaling Modulation
Abstract
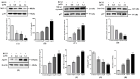
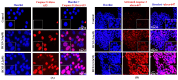
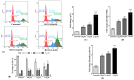
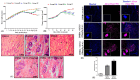
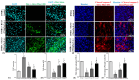
Background: Breast cancer influences more than 2 million women worldwide annually. Since apoptotic dysregulation is a cancer hallmark, targeting apoptotic regulators encompasses strategic drug development for cancer therapy. One such class of apoptotic regulators is inhibitors of apoptosis proteins (IAP) which are a class of E3 ubiquitin ligases that actively function to support cancer growth and survival. Methods: The current study reports design, synthesis, docking analysis (based on binding to IAP-BIR3 domains), anti-proliferative and anti-tumor potential of the azomethine derivative, 1-(4-chlorophenyl)-N-(4-ethoxyphenyl)methanimine (BCS3) on breast cancer (in vitro and in vivo) and its possible mechanisms of action. Results: Strong selective cytotoxic activity was observed in MDA-MB-231, MCF-7, and MDA-MB-468 breast cancer cell lines that exhibited IC50 values, 1.554 µM, 5.979 µM, and 6.462 µM, respectively, without affecting normal breast cells, MCF-10A. For the evaluation of the cytotoxic potential of BCS3, immunofluorescence, immunoblotting, and FACS (apoptosis and cell cycle) analyses were conducted. BCS3 antagonized IAPs, thereby causing MDM2-p53 and Bcl-2-Caspase-mediated intrinsic and extrinsic apoptosis. It also modulated p53 expression causing p21-CDK1/cyclin B1-mediated cell cycle arrest at S and G2/M phases. The in vitro findings were consistent with in vivo findings as observed by reduced tumor volume and apoptosis initiation (TUNEL assay) by IAP downregulation. BCS3 also produced potent synergistic effects with doxorubicin on tumor inhibition. Conclusions: Having witnessed the profound anti-proliferative potential of BCS3, the possible adverse effects related to anti-cancer therapy were examined following OECD 407 guidelines which confirmed its systemic safety profile and well tolerability. The results indicate the promising effect of BCS3 as an IAP antagonist for breast cancer therapy with fewer adverse effects.
Keywords: 7,12-dimethylbenz(a)anthracene; apoptosis; breast cancer; caspases; inhibitors of apoptosis protein; molecular docking.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

