Low-Dose Oral Ginger Improves Daily Symptom Scores in Asthma
- PMID: 39770492
- PMCID: PMC11728807
- DOI: 10.3390/ph17121651
Low-Dose Oral Ginger Improves Daily Symptom Scores in Asthma
Abstract
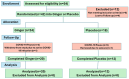
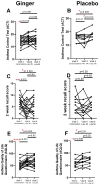
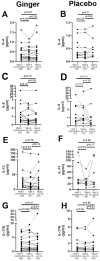
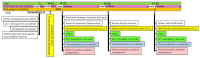
Background/Objective: A significant number of individuals with asthma have poorly controlled daily symptoms and utilize dietary supplements such as ginger in a quest for improved symptom control; however, its effectiveness at improving the control of symptoms is unproven. We questioned whether low-dose oral ginger would improve subjective and objective measurements of asthma control in mild-to-moderate asthmatics. Methods: We performed a randomized, placebo-controlled, double-blinded study of a low dose (1 g twice daily) of a dietary supplement of ginger in 32 mild-to-moderate uncontrolled asthmatics over a 2-month trial period while maintaining daily conventional asthma therapies. The planned primary outcomes included an increased tolerance to inhaled methacholine and decreased concentrations of fractional excretion of exhaled nitric oxide (FeNO). Secondary planned outcomes included measurements of asthma control by the Asthma Control Test (ACT), a 2-week symptom recall test, and the Juniper mini Asthma Quality of Life Questionnaire (AQLQ), and blood eosinophils and asthma-associated cytokines. Results: Exhaled nitric oxide or blood eosinophils were not changed by oral ginger. However, three different measures of asthma symptom control were improved by the 28-day time point of oral ginger. Asthma-associated serum cytokines (IL-13 and IL-17A) were modulated by oral ginger. Conclusions: This is the first demonstration that a small daily dose of a dietary supplement of ginger may improve asthma symptoms and reduce inflammation in human asthmatics. These findings support the need for additional studies using larger doses of ginger in specific endotypes of asthmatics that may identify a novel therapeutic for asthma.
Keywords: 2-week symptom recall; AQLQ; asthma control test; cytokines; eosinophils; exhaled nitric oxide; methacholine.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- CDC Most Recent National Asthma Data. [(accessed on 1 February 2023)]; Available online: https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm.
-
- Martin R.J., Szefler S.J., King T.S., Kraft M., Boushey H.A., Chinchilli V.M., Craig T.J., DiMango E.A., Deykin A., Fahy J.V., et al. The Predicting Response to Inhaled Corticosteroid Efficacy (PRICE) trial. J. Allergy Clin. Immunol. 2007;119:73–80. doi: 10.1016/j.jaci.2006.10.035. - DOI - PMC - PubMed
-
- AsthmaStats: Uncontrolled Asthma Among Persons with Current Asthma. [(accessed on 1 February 2023)];2014 Available online: https://archive.cdc.gov/www_cdc_gov/asthma/asthma_stats/uncontrolled_ast....
Grants and funding
LinkOut - more resources
Full Text Sources

