The Small GTPase Ran Increases Sensitivity of Ovarian Cancer Cells to Oncolytic Vesicular Stomatitis Virus
- PMID: 39770503
- PMCID: PMC11677601
- DOI: 10.3390/ph17121662
The Small GTPase Ran Increases Sensitivity of Ovarian Cancer Cells to Oncolytic Vesicular Stomatitis Virus
Abstract
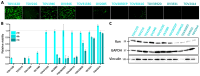
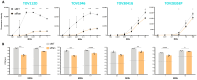
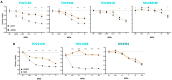
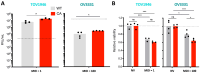
Background/Objectives: Ovarian cancer is the deadliest gynecologic cancer, and with the majority of patients dying within the first five years of diagnosis, new therapeutic options are required. The small guanosine triphosphatase (GTPase) Ras-related nuclear protein (Ran) has been reported to be highly expressed in high-grade serous ovarian cancers (HGSOCs) and associated with poor outcomes. Blocking Ran function or preventing its expression were shown to be promising treatment strategies, however, there are currently no small molecule inhibitors available to specifically inhibit Ran function. Interestingly, a previous study suggested that the Vesicular stomatitis virus (VSV) could inhibit Ran activity. Given that VSV is an oncolytic virus (OV) and, therefore, has anti-cancer activity, we reasoned that oncolytic VSV (oVSV) might be particularly effective against ovarian cancer via Ran inhibition. Methods: We evaluated the sensitivity of patient-derived ovarian cancer cell lines to oVSV, as well as the impact of oVSV on Ran and vice versa, using overexpression systems, small interfering RNAs (siRNAs), and drug inhibition. Results: In this study, we evaluated the interplay between oVSV and Ran and found that, although oVSV does not consistently block Ran, increased Ran activation allows for better oVSV replication and tumor cell killing. Conclusions: Our study reveals a positive impact of Ran on oVSV sensitivity. Given the high expression of Ran in HGSOCs, which are particularly aggressive ovarian cancers, our data suggest that oVSV could be effective against the deadliest form of the disease.
Keywords: RanGTP; VSV; oncolytic viruses; ovarian cancer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Oncolytic vesicular stomatitis virus alone or in combination with JAK inhibitors is effective against ovarian cancer.Mol Ther Oncol. 2024 Jun 8;32(3):200826. doi: 10.1016/j.omton.2024.200826. eCollection 2024 Sep 19. Mol Ther Oncol. 2024. PMID: 39006945 Free PMC article.
-
Intertumoral heterogeneity impacts oncolytic vesicular stomatitis virus efficacy in mouse pancreatic cancer cells.J Virol. 2023 Sep 28;97(9):e0100523. doi: 10.1128/jvi.01005-23. Epub 2023 Sep 6. J Virol. 2023. PMID: 37671865 Free PMC article.
-
Natural killer T cell immunotherapy combined with oncolytic vesicular stomatitis virus or reovirus treatments differentially increases survival in mouse models of ovarian and breast cancer metastasis.J Immunother Cancer. 2021 Mar;9(3):e002096. doi: 10.1136/jitc-2020-002096. J Immunother Cancer. 2021. PMID: 33722907 Free PMC article.
-
VSV based virotherapy in ovarian cancer: the past, the present and …future?J Cancer. 2017 Jul 22;8(12):2369-2383. doi: 10.7150/jca.19473. eCollection 2017. J Cancer. 2017. PMID: 28819441 Free PMC article. Review.
-
Vesicular stomatitis virus as a flexible platform for oncolytic virotherapy against cancer.J Gen Virol. 2012 Dec;93(Pt 12):2529-2545. doi: 10.1099/vir.0.046672-0. Epub 2012 Oct 10. J Gen Virol. 2012. PMID: 23052398 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

