Comparative Analyses of the Safety Profiles of Vitamin D Receptor Agonists: A Pharmacovigilance Study Based on the EudraVigilance Database
- PMID: 39770528
- PMCID: PMC11677518
- DOI: 10.3390/ph17121686
Comparative Analyses of the Safety Profiles of Vitamin D Receptor Agonists: A Pharmacovigilance Study Based on the EudraVigilance Database
Abstract
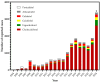
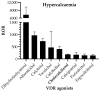
Background/Objectives: Vitamin D receptor (VDR) agonists are commonly used in clinical practice for their roles in calcium regulation and potential benefits in various diseases. However, their safety profiles, particularly for compounds available as food supplements, remain underexplored in real-world settings. This study aimed to analyze the safety profiles of VDR agonists using the EudraVigilance database, focusing on adverse drug reactions (ADRs) reported between 1 January 2004 and 23 June 2024. Methods: Data for ten VDR agonists were collected, de-duplicated, and analyzed to identify specific safety signals. Risk factors for specific ADRs were assessed using multiple logistic regression. Results: This study analyzed 5,369,581 reports in the EudraVigilance system, from which 17,947 reports (0.33%) involving 80,050 ADRs were linked to VDR agonists. The most-reported drugs were cholecalciferol (12,944 cases) and calcitriol (1355 cases). Serious ADRs were more prevalent with paricalcitol, alfacalcidol, and calcitriol than with cholecalciferol (p < 0.05). Hypercalcemia was a hallmark ADR for all VDR agonists, with the highest risk linked to dihydrotachysterol (ROR = 5668; 95%CI = 3332 to 9641; p < 0.0001), alfacalcidol (ROR = 965.7; 95%CI = 843.6 to 1106; p < 0.0001), and calcitriol (ROR = 726.0; 95%CI = 634.6 to 830.5; p < 0.0001). Logistic regression highlighted dehydration, overdose, and concomitant administration of calcium salts as major predictors of hypercalcemia. The co-administration of multiple VDR agonists was also found to increase hypercalcemia risk. However, the disproportionality analysis showed that only active VDR agonists (e.g., calcitriol, alfacalcidol) were associated with severe complications like renal and urinary disorders and cardiac issues due to hypercalcemia. Natural precursors (cholecalciferol, ergocalciferol) were more often linked to non-calcemic ADRs such as gastrointestinal symptoms, which were more prevalent in infants and children compared to adults. Conclusions: The safety profiles of VDR agonists differ significantly between compounds. Active derivatives require close monitoring for serious calcemia-related complications, whereas cholecalciferol is associated with less severe ADRs, primarily in at-risk populations. These findings highlight the need for targeted safety monitoring and further research into the real-world uses of VDR agonists.
Keywords: calcitriol; pharmacovigilance; safety profile; vitamin D; vitamin D receptor agonists.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Condoleo V., Pelaia C., Armentaro G., Severini G., Clausi E., Cassano V., Miceli S., Fiorentino T.V., Succurro E., Arturi F., et al. Role of Vitamin D in Cardiovascular Diseases. Endocrines. 2021;2:417–426. doi: 10.3390/endocrines2040037. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

