Immunoconjugates as an Efficient Platform for Drug Delivery: A Resurgence of Natural Products in Targeted Antitumor Therapy
- PMID: 39770542
- PMCID: PMC11677665
- DOI: 10.3390/ph17121701
Immunoconjugates as an Efficient Platform for Drug Delivery: A Resurgence of Natural Products in Targeted Antitumor Therapy
Abstract
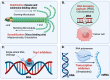
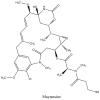
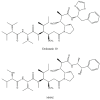
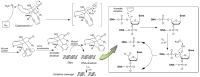
The present review provides a detailed and comprehensive discussion on antibody-drug conjugates (ADCs) as an evolving new modality in the current therapeutic landscape of malignant diseases. The principle concepts of targeted delivery of highly toxic agents forsaken as stand-alone drugs are examined in detail, along with the biochemical and technological tools for their successful implementation. An extensive analysis of ADCs' major components is conducted in parallel with their function and impact on the stability, efficacy, safety, and resistance profiles of the immunoconjugates. The scope of the article covers the major classes of currently validated natural compounds used as payloads, with an emphasis on their structural and mechanistic features, natural origin, and distribution. Future perspectives in ADCs' design are thoroughly explored, addressing their inherent or emerging challenges and limitations. The survey also provides a comprehensive overview of the molecular rationale for active tumor targeting of ADC-based platforms, exploring the cellular biology and clinical relevance of validated tumor markers used as a "homing" mechanism in both hematological and solid tumor malignancies.
Keywords: ADC; C-MET; HER2; Nectin-4; TROP-2; amatoxins; calicheamicins; camptothecins; dolastatins; drug delivery; immunotoxins; maytansinoids; pan-B-cell antigens; targeted antitumor therapy; tissue factor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Spotlight on ideal target antigens and resistance in antibody-drug conjugates: Strategies for competitive advancement.Drug Resist Updat. 2024 Jul;75:101086. doi: 10.1016/j.drup.2024.101086. Epub 2024 Apr 23. Drug Resist Updat. 2024. PMID: 38677200 Review.
-
Antibody-Drug Conjugates in Solid Tumor Oncology: An Effectiveness Payday with a Targeted Payload.Pharmaceutics. 2023 Aug 19;15(8):2160. doi: 10.3390/pharmaceutics15082160. Pharmaceutics. 2023. PMID: 37631374 Free PMC article. Review.
-
Leveraging TROP2 Antibody-Drug Conjugates in Solid Tumors.Annu Rev Med. 2024 Jan 29;75:31-48. doi: 10.1146/annurev-med-071322-065903. Epub 2023 Sep 27. Annu Rev Med. 2024. PMID: 37758237 Review.
-
Comparative Analysis and Future Prospects of Human Epidermal Growth Factor Receptor 2 (HER2) and Trophoblast Cell-Surface Antigen 2 (Trop-2) Targeted Antibody-Drug Conjugates in Breast Cancer Treatment.Breast Cancer (Dove Med Press). 2024 Sep 17;16:621-630. doi: 10.2147/BCTT.S480796. eCollection 2024. Breast Cancer (Dove Med Press). 2024. PMID: 39310781 Free PMC article. Review.
-
Antibody-Drug Conjugates in Breast Cancer: What Is Beyond HER2?Cancer J. 2022 Nov-Dec 01;28(6):436-445. doi: 10.1097/PPO.0000000000000629. Cancer J. 2022. PMID: 36383906 Review.
References
-
- Samantasinghar A., Sunildutt N.P., Ahmed F., Soomro A.M., Salih A.R.C., Parihar P., Memon F.H., Kim K.H., Kang I.S., Choi K.H. A Comprehensive Review of Key Factors Affecting the Efficacy of Antibody Drug Conjugate. Biomed. Pharmacother. 2023;161:114408. doi: 10.1016/j.biopha.2023.114408. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

