Antimicrobial Activity of Naphthyridine Derivatives
- PMID: 39770547
- PMCID: PMC11678664
- DOI: 10.3390/ph17121705
Antimicrobial Activity of Naphthyridine Derivatives
Abstract
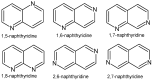
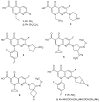
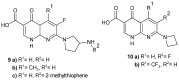
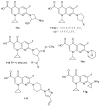
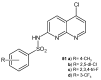
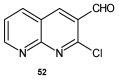
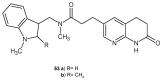
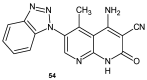
To combat the problem of the increasing drug resistance of microorganisms, it is necessary to constantly search for new medicinal substances that will demonstrate more effective mechanisms of action with a limited number of side effects. Naphthyridines are N-heterocyclic compounds containing a fused system of two pyridine rings, occurring in the form of six structural isomers with different positions of nitrogen atoms, which exhibit a wide spectrum of pharmacological activity, in particular antimicrobial properties. This review presents most of the literature data about the synthetic and natural naphthyridine derivatives that have been reported to possess antimicrobial activity.
Keywords: antimicrobial activity; nalidixic acid; naphthyridine derivatives; structure–activity relationships.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
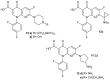
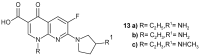
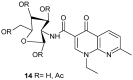
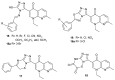
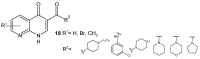
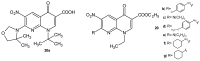
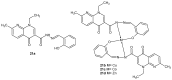
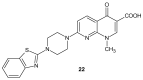
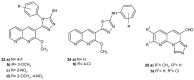
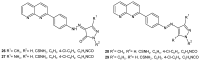
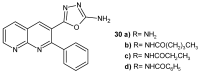

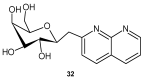
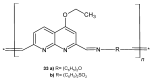
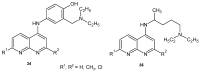
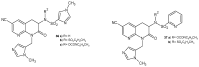
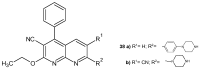
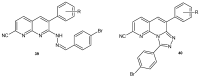
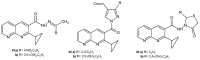
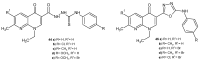
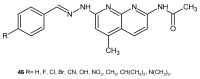
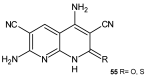
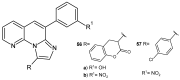
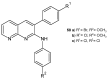
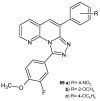
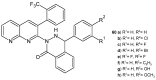
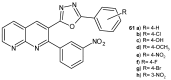
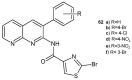
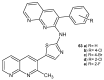
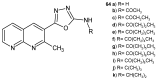
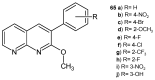
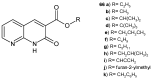
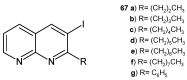
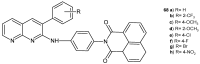
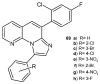
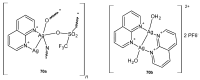
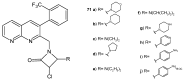
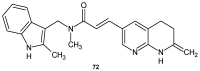
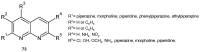
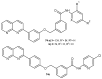
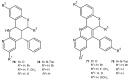
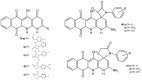
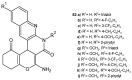
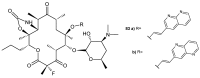
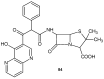
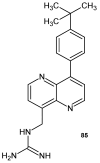
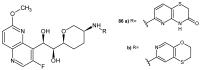
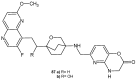
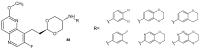
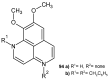
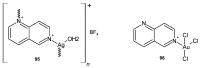
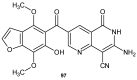
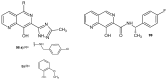
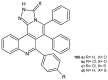
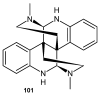
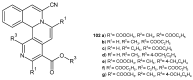
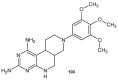
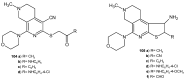
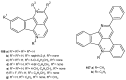
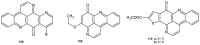
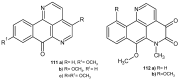
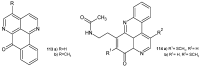
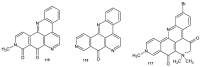
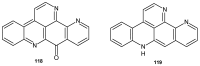
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

