Encapsulation of Nanocrystals in Mannitol-Based Inhalable Microparticles via Spray-Drying: A Promising Strategy for Lung Delivery of Curcumin
- PMID: 39770549
- PMCID: PMC11676507
- DOI: 10.3390/ph17121708
Encapsulation of Nanocrystals in Mannitol-Based Inhalable Microparticles via Spray-Drying: A Promising Strategy for Lung Delivery of Curcumin
Abstract
Background/objectives: Curcumin is well known for its great anti-inflammatory and antioxidant efficacy, representing a potential strategy for the treatment of respiratory disorders. However, several drawbacks, such as chemical instability, poor water solubility and rapid metabolism, result in low bioavailability, limiting its clinical applications. In this study, curcumin nanocrystals were incorporated into mannitol-based microparticles to obtain an inhalable dry powder.
Methods: A curcumin nanosuspension was produced by wet-ball media milling and thoroughly characterized. Spray drying was then used to produce mannitol microparticles incorporating curcumin nanocrystals. In vitro release/dissolution tests were carried out in simulated lung fluids, and the aerosolization properties were evaluated using a Next-Generation Impactor (NGI, Apparatus E Ph. Eu.).
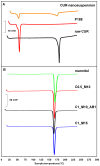
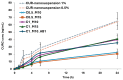
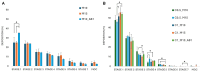
Results: The incorporation of curcumin nanocrystals into mannitol-based microparticles influenced their morphological properties, such as geometric diameters, and flowability. Despite these changes, nebulization studies confirmed optimal MMAD values (<5 µm), while multi-step dissolution/release studies evidenced the influence of mannitol.
Conclusions: The developed curcumin nanocrystals-loaded mannitol microparticles show promise as an inhalable treatment for respiratory diseases, combining effective aerodynamic properties with controlled drug release.
Keywords: curcumin; inhalable microparticles; nano into micro; nanosuspension; pulmonary delivery.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Nanocrystal Agglomerates of Curcumin Prepared by Electrospray Drying as an Excipient-Free Dry Powder for Inhalation.Adv Pharmacol Pharm Sci. 2024 Sep 6;2024:6288621. doi: 10.1155/2024/6288621. eCollection 2024. Adv Pharmacol Pharm Sci. 2024. PMID: 39281030 Free PMC article.
-
Pulmonary Delivery of Curcumin and Beclomethasone Dipropionate in a Multicomponent Nanosuspension for the Treatment of Bronchial Asthma.Pharmaceutics. 2021 Aug 20;13(8):1300. doi: 10.3390/pharmaceutics13081300. Pharmaceutics. 2021. PMID: 34452261 Free PMC article.
-
Preparation and in vivo absorption evaluation of spray dried powders containing salmon calcitonin loaded chitosan nanoparticles for pulmonary delivery.Drug Des Devel Ther. 2013 Aug 28;7:861-73. doi: 10.2147/DDDT.S47681. eCollection 2013. Drug Des Devel Ther. 2013. PMID: 24039397 Free PMC article.
-
A Review on Micro and Nanoengineering in Powder-Based Pulmonary Drug Delivery.Int J Pharm. 2024 Jun 25;659:124248. doi: 10.1016/j.ijpharm.2024.124248. Epub 2024 May 21. Int J Pharm. 2024. PMID: 38782150 Review.
-
Current Research on Spray-Dried Chitosan Nanocomposite Microparticles for Pulmonary Drug Delivery.Pharm Nanotechnol. 2023;11(2):127-137. doi: 10.2174/2211738511666221128093822. Pharm Nanotechnol. 2023. PMID: 36443972 Review.
Cited by
-
Inhalable Nanotechnology-Based Drug Delivery Systems for the Treatment of Inflammatory Lung Diseases.Pharmaceutics. 2025 Jul 9;17(7):893. doi: 10.3390/pharmaceutics17070893. Pharmaceutics. 2025. PMID: 40733101 Free PMC article. Review.
References
-
- Santana F.P.R., Pinheiro N.M., Mernak M.I.B., Righetti R.F., Martins M.A., Lago J.H.G., Lopes F.D.T.Q.D.S., Tibério I.F.L.C., Prado C.M. Evidences of herbal medicine-derived natural products effects in inflammatory lung diseases. Mediators Inflamm. 2016;2016:2348968. doi: 10.1155/2016/2348968. - DOI - PMC - PubMed
-
- Rahman M.M., Bibi S., Rahaman M.S., Rahman F., Islam F., Khan M.S., Hasan M.M., Parvez A., Hossain M.A., Maeesa S.K., et al. Natural therapeutics and nutraceuticals for lung diseases: Traditional significance, phytochemistry, and pharmacology. Biomed. Pharmacother. 2022;150:113041. doi: 10.1016/j.biopha.2022.113041. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

