The Interaction of Histamine H3 and Dopamine D1 Receptors on Hyperkinetic Alterations in Animal Models of Parkinson's Disease
- PMID: 39770568
- PMCID: PMC11679969
- DOI: 10.3390/ph17121726
The Interaction of Histamine H3 and Dopamine D1 Receptors on Hyperkinetic Alterations in Animal Models of Parkinson's Disease
Abstract
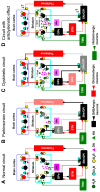
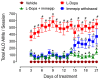
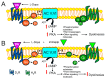
Parkinson's disease is associated with the loss of more than 40% of dopaminergic neurons in the substantia nigra pars compacta. One of the therapeutic options for restoring striatal dopamine levels is the administration of L-3,4-dihydroxyphenylalanine (L-Dopa). However, Parkinson's disease patients on long-term L-Dopa therapy often experience motor complications, such as dyskinesias. L-Dopa-induced dyskinesias (LIDs) manifest as abnormal involuntary movements and are produced by elevated striatal dopamine levels, which lead to increased activity of the basal ganglia direct striato-nigral pathway. Dopamine D1 receptors are more than 95% confined to neurons of the direct pathway, where they colocalize with histamine H3 receptors. There is evidence of functional interactions between D1 and H3 receptors, and here we review the consequences of these interactions on LIDs.
Keywords: D1 receptor; GABA; H3 receptor; L-Dopa-induced dyskinesias; Parkinson’s disease; cerebral cortex; dopamine; glutamate; histamine; striatum.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

