Pentagalloyl Glucose from Bouea macrophylla Suppresses the Epithelial-Mesenchymal Transition and Synergizes the Doxorubicin-Induced Anticancer and Anti-Migration Effects in Triple-Negative Breast Cancer
- PMID: 39770571
- PMCID: PMC11679756
- DOI: 10.3390/ph17121729
Pentagalloyl Glucose from Bouea macrophylla Suppresses the Epithelial-Mesenchymal Transition and Synergizes the Doxorubicin-Induced Anticancer and Anti-Migration Effects in Triple-Negative Breast Cancer
Abstract
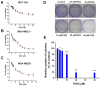
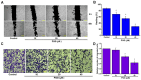
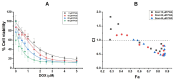
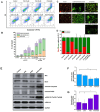
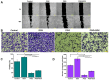
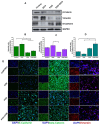
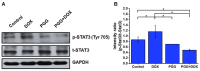
Background: Triple-negative breast cancer (TNBC) represents an aggressive form of breast cancer with few available therapeutic options. Chemotherapy, particularly with drugs like doxorubicin (DOX), remains the cornerstone of treatment for this challenging subtype. However, the clinical utility of DOX is hampered by adverse effects that escalate with higher doses and drug resistance, underscoring the need for alternative therapies. This study explored the efficacy of pentagalloyl glucose (PGG), a natural polyphenol derived from Bouea macrophylla, in enhancing DOX's anticancer effects and suppressing the epithelial-mesenchymal transition (EMT) in TNBC cells. Methods: This study employed diverse methodologies to assess the effects of PGG and DOX on TNBC cells. MDA-MB231 triple-negative breast cancer cells were used to evaluate cell viability, migration, invasion, apoptosis, mitochondrial membrane potential, and protein expression through techniques including MTT assays, wound healing assays, flow cytometry, Western blotting, and immunofluorescence. Results: Our findings demonstrate that PGG combined with DOX significantly inhibits TNBC cell proliferation, migration, and invasion. PGG enhances DOX-induced apoptosis by disrupting the mitochondrial membrane potential and activating caspase pathways; consequently, the activation of caspase-3 and the cleavage of PARP are increased. Additionally, the study shows that the combination treatment upregulates ERK signaling, further promoting apoptosis. Moreover, PGG reverses DOX-induced EMT by downregulating mesenchymal markers (vimentin and β-catenin) and upregulating epithelial markers (E-cadherin). Furthermore, it effectively inhibits STAT3 phosphorylation, associated with cell survival and migration. Conclusions: These results highlight the potential of PGG as an adjuvant therapy in TNBC treatment. PGG synergizes with DOX, which potentiates its anticancer effects while mitigating adverse reactions.
Keywords: doxorubicin; epithelial–mesenchymal transition (EMT); pentagalloyl glucose (PGG); synergistic; triple-negative breast cancer (TNBC).
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; the collection, analysis, or interpretation of data; the writing of the manuscript; or the decision to publish the results.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

