Aerosol Inhalation of Luteolin-7-O-Glucuronide Exerts Anti-Inflammatory Effects by Inhibiting NLRP3 Inflammasome Activation
- PMID: 39770573
- PMCID: PMC11677241
- DOI: 10.3390/ph17121731
Aerosol Inhalation of Luteolin-7-O-Glucuronide Exerts Anti-Inflammatory Effects by Inhibiting NLRP3 Inflammasome Activation
Abstract
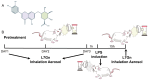
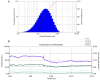
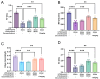
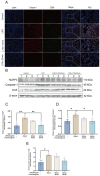
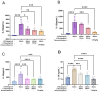
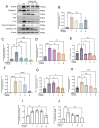
Background: Luteolin-7-O-glucuronide (L7Gn) is a flavonoid isolated from numerous traditional Chinese herbal medicines that exerts anti-inflammatory effects. Previous research has revealed that aerosol inhalation is the most straightforward way of administration for the delivery of respiratory agents. Thus far, the impact of aerosol inhalation of L7Gn on lung inflammation and the underlying mechanisms remain unknown. Methods: The real-time particle size for L7Gn aerosol inhalation was detected by the Spraytec spray droplet size measurement system, including transmission and size diameters. The acute lung injury (ALI) rat model was induced by aerosol inhalation of LPS to evaluate the protective effect of L7Gn. The inhibitory effect of NLRP3 inflammasome activation assays was conducted in LPS-induced MH-S cells. Elisa, Western blotting, and RT-PCR were utilized to investigate the expression of NLRP3 inflammasome-relevant proteins and genes. Results: In this study, we found that inhalation of L7Gn aerosol significantly reduced pulmonary injury by inhibiting inflammatory infiltration and enhancing lung function. Meanwhile, the NLR family pyrin domain containing 3 (NLRP3) inflammasome was activated dramatically, accompanied by upregulated expression of IL-1β and IL-18, both in the ALI rat model and in LPS-induced MH-S cells. Moreover, L7Gn was found to significantly downregulate the expression of NLRP3, ASC, caspase-1, and cleaved caspase-1, which are critical components of the NLRP3 inflammasome, as well as the expression of IL-1β and IL-18. Conclusions: Based on our findings, L7Gn could exert anti-inflammatory effects by inhibiting NLRP3 inflammasome activation, which may emerge as potential therapeutic agents for the treatment of ALI.
Keywords: Inflammation inhibition; NLRP3 inflammasome; acute lung injury; aerosol delivery; luteolin-7-O-glucuronide.
Conflict of interest statement
The authors declare that there are no conflicts of interest in relation to this work.
Figures

References
-
- Xian H., Liu Y., Rundberg Nilsson A., Gatchalian R., Crother T.R., Tourtellotte W.G., Zhang Y., Aleman-Muench G.R., Lewis G., Chen W., et al. Metformin Inhibition of Mitochondrial ATP and DNA Synthesis Abrogates NLRP3 Inflammasome Activation and Pulmonary Inflammation. Immunity. 2021;54:1463–1477. doi: 10.1016/j.immuni.2021.05.004. - DOI - PMC - PubMed
-
- de Souza Xavier Costa N., Ribeiro Júnior G., dos Santos Alemany A.A., Belotti L., Zati D.H., Frota Cavalcante M., Matera Veras M., Ribeiro S., Kallás E.G., Nascimento Saldiva P.H., et al. Early and Late Pulmonary Effects of Nebulized LPS in Mice: An Acute Lung Injury Model. PLoS ONE. 2017;12:e0185474. doi: 10.1371/journal.pone.0185474. - DOI - PMC - PubMed
Grants and funding
- C12021A04615/Science and Technology Innovation Project of the Chinese Academy of Traditional Chinese Medicine
- CI2021A04802/Science and Technology Innovation Project of the Chinese Academy of Traditional Chinese Medicine
- CI2021B015/Scientific and Technological Innovation Project of China Academy of Chinese Medical Sciences
- 82204740/National Natural Science Fund Youth Fund of China
- 82104502/National Natural Science Fund Youth Fund of China
LinkOut - more resources
Full Text Sources
Miscellaneous

