Safety Evaluation of the Combination with Dexrazoxane and Anthracyclines: A Disproportionality Analysis Based on the Food and Drug Administration Adverse Event Reporting System Database
- PMID: 39770581
- PMCID: PMC11678267
- DOI: 10.3390/ph17121739
Safety Evaluation of the Combination with Dexrazoxane and Anthracyclines: A Disproportionality Analysis Based on the Food and Drug Administration Adverse Event Reporting System Database
Abstract
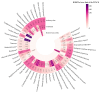
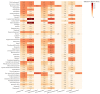
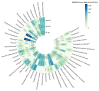
Objectives: As one of the important interventions to alleviate anthracycline-related cardiotoxicity (ARC), the safety assessment of dexrazoxane in clinical practice is particularly important. This study aims to evaluate the actual efficacy and potential adverse effects of dexrazoxane in clinical practice by analyzing the reports of adverse events (AEs) related to the combination with dexrazoxane and anthracyclines. Methods: We utilized four disproportionality analysis methods to analyze AE reports of the combination with dexrazoxane and anthracyclines in the Food and Drug Administration Adverse Event Reporting System (FAERS) database from the third quarter of 2014 to the first quarter of 2024. Results: Under the three backgrounds, a large number of preferred terms (PTs) such as cardiac failure disappeared in the combined group, and the PTs with significant signal values were mainly concentrated in infections and infestations. For patients under 18, some PTs associated with infections and infestations disappeared after the combination of the two drugs. Conclusions: Dexrazoxane can effectively alleviate ARC, but it may also increase the risk of infection. For infections and infestations, children under 18 years old are more likely to benefit from the combination therapy. More attention should be paid to infectious AEs in the clinical use of dexrazoxane, though disproportionality analysis is a hypothesis-generating approach.
Keywords: anthracyclines; cardiotoxicity; children; dexrazoxane; disproportionality analysis; infection.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Lyon A.R., López-Fernández T., Couch L.S., Asteggiano R., Aznar M.C., Bergler-Klein J., Boriani G., Cardinale D., Cordoba R., Cosyns B., et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS) Eur. Heart J. Cardiovasc. Imaging. 2022;23:e333–e465. doi: 10.1093/ehjci/jeac106. - DOI - PubMed
-
- Li X., Li Y., Zhang T., Xiong X., Liu N., Pang B., Ruan Y., Gao Y., Shang H., Xing Y. Role of cardioprotective agents on chemotherapy-induced heart failure: A systematic review and network meta-analysis of randomized controlled trials. Pharmacol. Res. 2020;151:104577. doi: 10.1016/j.phrs.2019.104577. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

