Bacillus lumedeiriae sp. nov., a Gram-Positive, Spore-Forming Rod Isolated from a Pharmaceutical Facility Production Environment and Added to the MALDI Biotyper® Database
- PMID: 39770710
- PMCID: PMC11728281
- DOI: 10.3390/microorganisms12122507
Bacillus lumedeiriae sp. nov., a Gram-Positive, Spore-Forming Rod Isolated from a Pharmaceutical Facility Production Environment and Added to the MALDI Biotyper® Database
Abstract
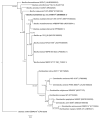
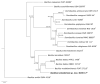
A Gram-positive, aerobic, rod-shaped and spore-forming bacterium strain designation, B190/17, was isolated from an air monitoring sample of a Brazilian immunobiological production facility in 2017. The strain was not identifiable by biochemical methodology VITEK® 2 or by MALDI-TOF MS with VITEK® MS RUO and MALDI Biotyper®. The 16S rRNA gene sequencing results showed 98.51% similarity with Bacillus wudalianchiensis FJAT 27215T, 98.28% with 'Bacillus aerolatus' CX 253T, 97.96% with Bacillus badius MTCC 1458T, 97.63% with Bacillus xiapuensis FJAT 46582T and 97.21% with Bacillus thermotolerans SGZ8T. Biochemical data showed that the strain was alanine arylamidase-, Ala-Phe-Pro arylamidase-, ELLMAN (cysteine residues)-, leucine arylamidase-, phenyalanine arylamidase- and tyrosine arylamidase-positive. The genomic DNA G+C% content of B190/17 was 41.6 mol%. The phylogenetic, genomic taxonomy and biochemical tests suggested that B190/17 represents a novel species and should be classified as the type strain of a novel Bacillus species. The name Bacillus lumedeiriae sp. nov. was proposed. After characterization, B190/17 was added to the MALDI Biotyper® database as Bacillus lumedeiriae sp. nov.
Keywords: 16S rRNA; Bacillus lumedeiriae; MALDI-TOF MS; bacterial identification; genomic taxonomy; pharmaceutical industry.
Conflict of interest statement
The author Stephen James Forsythe is the maintainer of Foodmicrobe.com Ltd. The remaining authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures

Similar articles
-
Characterization by MALDI-TOF MS and 16S rRNA Gene Sequencing of Aerobic Endospore-Forming Bacteria Isolated from Pharmaceutical Facility in Rio de Janeiro, Brazil.Microorganisms. 2024 Apr 3;12(4):724. doi: 10.3390/microorganisms12040724. Microorganisms. 2024. PMID: 38674668 Free PMC article.
-
Bacillus wudalianchiensis sp. nov., isolated from grass soils of the Wudalianchi scenic area.Int J Syst Evol Microbiol. 2017 Aug;67(8):2897-2902. doi: 10.1099/ijsem.0.002042. Epub 2017 Aug 18. Int J Syst Evol Microbiol. 2017. PMID: 28820124
-
Bacillus xiapuensis sp. nov., isolated from marine sediment.Int J Syst Evol Microbiol. 2019 Jun;69(6):1714-1719. doi: 10.1099/ijsem.0.003381. Epub 2019 Apr 4. Int J Syst Evol Microbiol. 2019. PMID: 30950781
-
Bacillus aerolatus sp. nov., a novel member of the genus Bacillus, isolated from bioaerosols in a school playground.Arch Microbiol. 2020 Nov;202(9):2373-2378. doi: 10.1007/s00203-020-01955-3. Epub 2020 Jun 24. Arch Microbiol. 2020. PMID: 32583126
-
Bacillus litorisediminis sp. nov., a Thermophilic Bacterium Isolated from Mangrove Sediment.Curr Microbiol. 2023 Jan 19;80(2):79. doi: 10.1007/s00284-023-03180-9. Curr Microbiol. 2023. PMID: 36656344
References
-
- Costa L.V.D., Miranda R.V.D.S.L., Reis C.M.F., Andrade J.M., Cruz F.V., Frazão A.M., Fonseca E.L., Ramos J.N., Brandão M.L.L., Vieira V.V. MALDI-TOF MS database expansion for identification of Bacillus and related genera isolated from a pharmaceutical facility. J. Microbiol. Methods. 2022;203:106625. doi: 10.1016/j.mimet.2022.106625. - DOI - PubMed
-
- Song M., Li Q., Liu C., Wang P., Qin F., Zhang L., Fan Y., Shao H., Chen G., Yang M. A comprehensive technology strategy for microbial identification and contamination investigation in the sterile drug manufacturing facility—A case study. Front. Microbiol. 2024;15:1327175. doi: 10.3389/fmicb.2024.1327175. - DOI - PMC - PubMed
-
- European Medicines Agency . The Rules Governing Medicinal Products in the European Union. Volume 4 European Medicines Agency; Brussels, Belgium: 2022. European Union guidelines for good manufacturing practice for medicinal products for human and veterinary use. Annex 1: Manufacture of Sterile Medicinal Products.
-
- Miranda R.V.D.S.L., da Costa L.V., Albuquerque L.S., Dos Reis C.M.F., Braga L.M.P.D.S., de Andrade J.M., Ramos J.N., Mattoso J.M.V., Forsythe S.J., Brandão M.L.L. Identification of Sutcliffiella horikoshii strains in an immunobiological pharmaceutical industry facility. Lett. Appl. Microbiol. 2023;76:ovad056. doi: 10.1093/lambio/ovad056. - DOI - PubMed
-
- Mattoso J.M.V., Costa L.V.C., Vale B.A., Reis C.M.F., Andrade J.M., Braga L.M.P.S., Conceição G.M.S., Costa P.B.M., Silva I.B., Rodrigues L.A.P., et al. Quantitative and qualitative evaluation of microorganism profile identified in bioburden analysis in a biopharmaceutical facility in Brazil: Criteria for classification and management of results. PDA J. Pharm. Sci. Technol. 2024;78 doi: 10.5731/pdajpst.2023.012883. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

