Enhanced Production of IL-10 in PCR-Positive Dogs Infected with E. canis and A. phagocytophilum Facilitate Specific Immune Responses
- PMID: 39770719
- PMCID: PMC11679728
- DOI: 10.3390/microorganisms12122516
Enhanced Production of IL-10 in PCR-Positive Dogs Infected with E. canis and A. phagocytophilum Facilitate Specific Immune Responses
Abstract
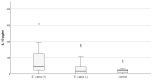
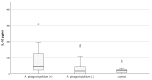
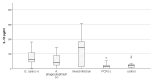
Infection of dogs with the tick-borne rickettsiae Ehrlichia and Anaplasma provokes an immune response mediating the pathology and bacterial resistance. IL-10 is the main anti-inflammatory cytokine and plays a multifaceted role in host protection. The study aimed to investigate circulating IL-10 in 32 dogs naturally infected with A. phagocytophilum and E. canis, identified by PCR positivity, and 33 PCR-negative animals which tested positive for antibodies against these pathogens, as well as 22 healthy animals. The highest quantity of IL-10, measured by ELISA, was observed among dogs positive simultaneously for anti-E. canis and anti-A. phagocytophilum IgG antibodies, followed by dogs positive for anti-E. canis only. The concentration of IL-10 in PCR-positive dogs was almost three and a half times higher than that measured in the control group (77.09 ± 23.61 pg./mL vs. 21.55 ± 4.61 pg./mL; p = 0.0015) and five times higher than the concentration of interleukin in PCR-negative animals (77.09 ± 23.61 pg./mL vs. 14.86 ± 3.01 pg./mL; p = 0.000016). The highest level of IL-10 was observed in PCR-positive dogs with mixed infection (120.54 ± 44.18), followed by the level in PCR-positive dogs for E. canis only (78.81 ± 16.92). The lowest level of IL-10 was observed in PCR-positive dogs for A. phagocytophilum only (56.32 ± 12.68). We may suggest that infection with E. canis and A. phagocytophilum stimulates the IL-10 production in dogs, which may facilitate specific antibody responses.
Keywords: A. phagocytophilum; Anaplasma; Anaplasmataceae; E. canis; Ehrlichia; IL-10; antibody synthesis; cytokine.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Seroprevalence of Anaplasma spp. and Ehrlichia spp. and molecular detection of Anaplasma phagocytophilum and Anaplasma platys in stray dogs in Bosnia and Herzegovina.Ticks Tick Borne Dis. 2022 Mar;13(2):101875. doi: 10.1016/j.ttbdis.2021.101875. Epub 2021 Dec 3. Ticks Tick Borne Dis. 2022. PMID: 34894522
-
Comparative Experimental Infection Study in Dogs with Ehrlichia canis, E. chaffeensis, Anaplasma platys and A. phagocytophilum.PLoS One. 2016 Feb 3;11(2):e0148239. doi: 10.1371/journal.pone.0148239. eCollection 2016. PLoS One. 2016. PMID: 26840398 Free PMC article.
-
Molecular detection of Anaplasma platys, Anaplasma phagocytophilum and Wolbachia sp. but not Ehrlichia canis in Croatian dogs.Parasitol Res. 2017 Nov;116(11):3019-3026. doi: 10.1007/s00436-017-5611-y. Epub 2017 Sep 14. Parasitol Res. 2017. PMID: 28905230
-
Detection of canine vector-borne diseases in eastern Poland by ELISA and PCR.Parasitol Res. 2016 Mar;115(3):1039-44. doi: 10.1007/s00436-015-4832-1. Epub 2015 Nov 19. Parasitol Res. 2016. PMID: 26581374 Free PMC article.
-
Diversity of Anaplasma and Ehrlichia/Neoehrlichia Agents in Terrestrial Wild Carnivores Worldwide: Implications for Human and Domestic Animal Health and Wildlife Conservation.Front Vet Sci. 2018 Nov 23;5:293. doi: 10.3389/fvets.2018.00293. eCollection 2018. Front Vet Sci. 2018. PMID: 30533417 Free PMC article. Review.
References
-
- Dahmani M., Davoust B., Sambou M., Bassene H., Scandola P., Ameur T., Raoult D., Fenollar F., Mediannikov O. Molecular investigation and phylogeny of species of the Anaplasmataceae infecting animals and ticks in Senegal. Parasit. Vectors. 2019;12:495. doi: 10.1186/s13071-019-3742-y. - DOI - PMC - PubMed
-
- Pantchev N., Schnyder M., Vrhovec M.G., Schaper R., Tsachev I. Current surveys of the seroprevalence of Borrelia burgdorferi, Ehrlichia canis, Anaplasma phagocytophilum, Leishmania infantum, Babesia canis, Angiostrongylus vasorum and Dirofilaria immitis in dogs in Bulgaria. Parasitol. Res. 2015;114:117. doi: 10.1007/s00436-015-4518-8. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

