Harnessing the Power of Our Immune System: The Antimicrobial and Antibiofilm Properties of Nitric Oxide
- PMID: 39770746
- PMCID: PMC11677572
- DOI: 10.3390/microorganisms12122543
Harnessing the Power of Our Immune System: The Antimicrobial and Antibiofilm Properties of Nitric Oxide
Abstract
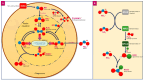
Nitric oxide (NO) is a free radical of the human innate immune response to invading pathogens. NO, produced by nitric oxide synthases (NOSs), is used by the immune system to kill microorganisms encapsulated within phagosomes via protein and DNA disruption. Owing to its ability to disperse biofilm-bound microorganisms, penetrate the biofilm matrix, and act as a signal molecule, NO may also be effective as an antibiofilm agent. NO can be considered an underappreciated antimicrobial that could be levied against infected, at-risk, and hard-to-heal wounds due to the inherent lack of bacterial resistance, and tolerance by human tissues. NO produced within a wound dressing may be an effective method of disrupting biofilms and killing microorganisms in hard-to-heal wounds such as diabetic foot ulcers, venous leg ulcers, and pressure injuries. We have conducted a narrative review of the evidence underlying the key antimicrobial and antibiofilm mechanisms of action of NO for it to serve as an exogenously-produced antimicrobial agent in dressings used in the treatment of hard-to-heal wounds.
Keywords: antimicrobial; biofilm; hard-to-heal; nitric oxide; wound.
Conflict of interest statement
Jonathan Matthew Roberts, Scarlet Milo, and Daniel Gary Metcalf declare employment and stock/stock options from Convatec.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

