Variations in the Bacterial, Fungal, and Protist Communities and Their Interactions Within Sediment Affected by the Benthic Organism, Snail Bellamya purificata
- PMID: 39770752
- PMCID: PMC11676288
- DOI: 10.3390/microorganisms12122550
Variations in the Bacterial, Fungal, and Protist Communities and Their Interactions Within Sediment Affected by the Benthic Organism, Snail Bellamya purificata
Abstract
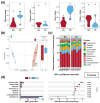
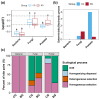
In aquatic benthic environments, benthic organisms have been found to regulate important biogeochemical characteristics and perform key ecosystem functions. To further explore the ecological impact of the snail Bellamya purificata's, presence on the benthic environment, we employed high-throughput sequencing technology to investigate its effects on the bacterial, fungal, and protist communities in sediment and their intrinsic interactions. Our findings revealed that B. purificata's presence significantly enhanced the diversity and evenness of the fungal community while simultaneously decreasing the diversity and richness of the protist community, and it also altered the composition and relative abundance of the dominant phyla across the bacterial, fungal, and protist communities. The snail B. purificata considerably altered the co-occurrence networks of the microbial communities, particularly by enhancing the intrinsic complexity of the protist community and by strengthening the interconnections among the protist, bacterial, and fungal communities. Notably, the proportions of specialists within the sediment bacterial, fungal, and protist communities declined due to the snail B. purificata. Its presence also notably expanded the habitat niche breadth for sediment bacteria and protists. In terms of community assembly, B. purificata shifted the fungal community assembly from being dominated by stochastic processes to being dominated by deterministic processes, whereas the protist community assembly shifted from deterministic processes to being dominated by stochastic processes. The mainly altered ecological processes in the fungal and protist assemblies were drift and homogenizing selection. Additionally, the presence of B. purificata resulted in a notable reduction in the sediment ON level and a significant increase in the ammonia, FA, and EN concentrations. Sediment properties, particularly FA and nitrate, were strongly correlated with microbial communities and were key contributors to changes in microbial community dynamics. These research findings not only broadened our understanding of the ecological impacts of B. purificata on benthic microbial communities but also highlighted its substantial potential in enhancing microbial community stability.
Keywords: Bellamya purificata; bacterial; fungal; microbial community; protist communities; sediment.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Norling K., Rosenberg R., Hulth S., Gremare A., Bonsdorff E. Importance of functional biodiversity and species-specific traits of benthic fauna for ecosystem functions in marine sediment. Mar. Ecol. Prog. Ser. 2007;332:11–23. doi: 10.3354/meps332011. - DOI
-
- Aller R.C. Benthic fauna and biogeochemical processes in marine sediments: The role of burrow structures. In: Blackburn T.H., Sorensen J., editors. Nitrogen Cycling in Coastal Marine Environments. John Wiley & Sons Ltd.; Hoboken, NJ, USA: 1988. pp. 301–338.
-
- Pelegrí S.P., Blackburn T.H. Bioturbation effects of the amphipod Corophium volutator on microbial nitrogen transformations in marine sediments. Mar. Biol. 1994;121:253–258. doi: 10.1007/BF00346733. - DOI
-
- Murray J.M.H., Meadows A., Meadows P.S. Biogeomorphological implications of microscale interactions between sediment geotechnics and marine benthos: A review. Geomorphology. 2002;47:15–30. doi: 10.1016/S0169-555X(02)00138-1. - DOI
-
- Stockdale A., Davison W., Zhang H. Micro-scale biogeochemical heterogeneity in sediments: A review of available technology and observed evidence. Earth-Sci. Rev. 2009;92:81–97. doi: 10.1016/j.earscirev.2008.11.003. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

